Concepts in Biochemistry
Upon completion of this chapter, the student will be able to achieve the following outcomes:
• Explain the role of biochemistry in dental hygiene and nutrition.
• Assign biomolecules according to functional group.
• Compare and contrast the structure, function, and properties of the four major classes of biomolecules (carbohydrates, proteins, nucleic acids, and lipids).
• Outline the structure, function, and property of monosaccharides, disaccharides, and polysaccharides.
• Outline the structure, function, and property of amino acids and proteins.
• Compare and contrast the roles of enzymes, coenzymes, and vitamins in nutrition.
• Outline the structure, function, and property of nucleotides and nucleic acids.
• Outline the structure, function, and property of fatty acids, triglycerides, and steroids.
• Differentiate catabolism from anabolism. Explain connections between metabolic pathways in carbohydrate, protein, and lipid metabolism.
Fundamentals of Biochemistry
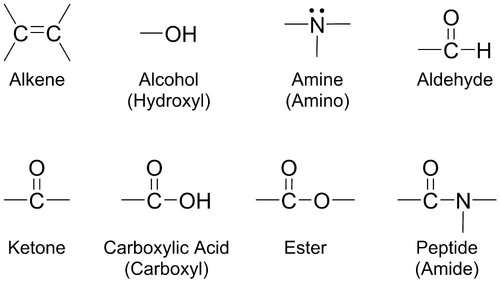
Atoms in a compound are held together by chemical bonds. There are two types of chemical bonds that form. An ionic bond forms between a positively charged metal ion and a negatively charged nonmetal ion. Hydroxyapatite in tooth enamel is composed of ionic bonds between calcium ions (Ca2+), phosphate ions (PO43−), and hydroxide ions (OH−). A covalent bond forms when electrons are equally shared between two nonmetals. Ultimately, the biomolecules responsible for life are based on carbon (C) because of carbon’s ability to form stable covalent bonds to itself and many other atoms, forming long chains and rings. In addition to carbon, the combination of different atoms, like hydrogen (H), oxygen (O), nitrogen (N), sulfur (S), and phosphorus (P), into biomolecules provides for great variety in chemical structure, properties and reactivity in biological systems. One way to organize this variety in chemical structure is the classification of molecules into functional groups. A functional group is a group of atoms that gives a family of molecules its characteristic chemical and physical properties. Molecules that have similar functional groups have similar properties. Figure 2-1 defines and exemplifies a few functional groups found in biochemistry.
Principle Biomolecules in Nutrition
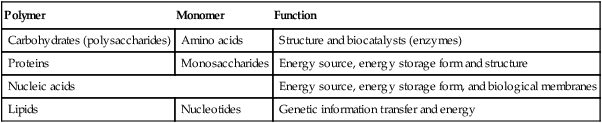
As shown in Table 2-1, the four major classes of biomolecules are carbohydrates, proteins, nucleic acids, and lipids. These biomolecules are characterized by the type of polymer, and monomer they contain as well as by their general function. A polymer is a large molecule containing numerous repeating units called monomers. A monomer is the smallest repeating unit present in a polymer.
Table 2-1
The four major classes of biomolecules
| Polymer | Monomer | Function |
| Carbohydrates (polysaccharides) | Amino acids | Structure and biocatalysts (enzymes) |
| Proteins | Monosaccharides | Energy source, energy storage form and structure |
| Nucleic acids | Energy source, energy storage form, and biological membranes | |
| Lipids | Nucleotides | Genetic information transfer and energy |
Carbohydrate Structure and Function
The biological function of carbohydrates involves energy metabolism and storage. As shown in Figure 2-2, plants use photosynthesis to make oxygen (O2) and glucose (C6H12O6), the carbohydrate from which animals acquire the energy required for life. Via the process of respiration, animals degrade the carbohydrate glucose (C6H12O6) into CO2 and water (H2O), and plants use these products for photosynthesis.

Carbohydrates are classified as monosaccharides, disaccharides, and polysaccharides, depending on the number of sugar monomers present (one, two, or many). Monosaccharides are composed of a single monomeric unit with the molecular formula Cn(H2O)n, where n is 3 to 8. Figure 2-3 shows the linear structures of the most common monosaccharides. Monosaccharides undergo oxidation-reduction reactions. When a monosaccharide is reduced, the aldehyde functional group changes to a sugar alcohol (e.g., sorbitol).
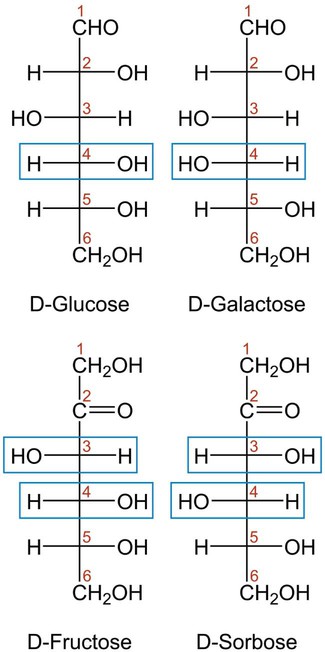
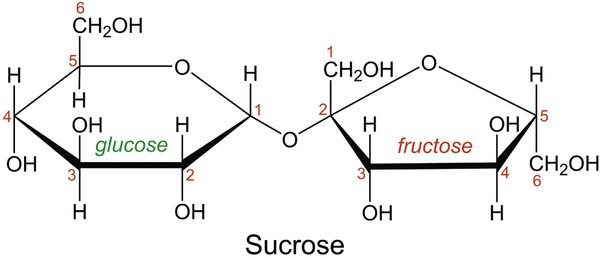
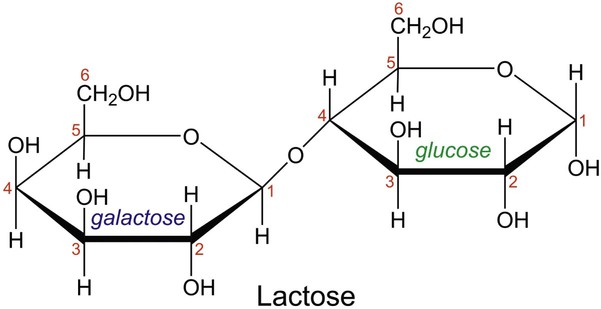
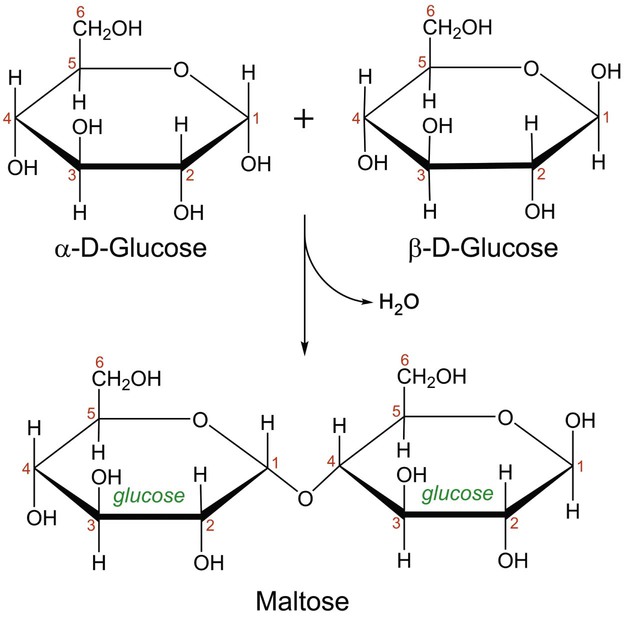
In aqueous solution, linear monosaccharides spontaneously form cyclic structures. When two monosaccharides combine, a disaccharide is formed. This involves the formation of a glycosidic bond. As shown in Figure 2-4, two glucose monomers can combine via a condensation reaction to form maltose. Maltose is a disaccharide that results from the degradation of starch and is used in brewing alcoholic beverages. When glucose and fructose combine, the disaccharide sucrose is formed (Figure 2-5). This disaccharide is table sugar and one of the sweetest carbohydrates. When the two monosaccharides galactose and glucose combine, the disaccharide lactose is formed (Figure 2-5). This disaccharide is found in milk and dairy products.
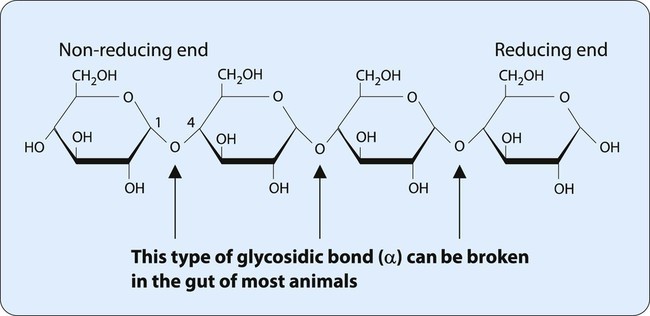
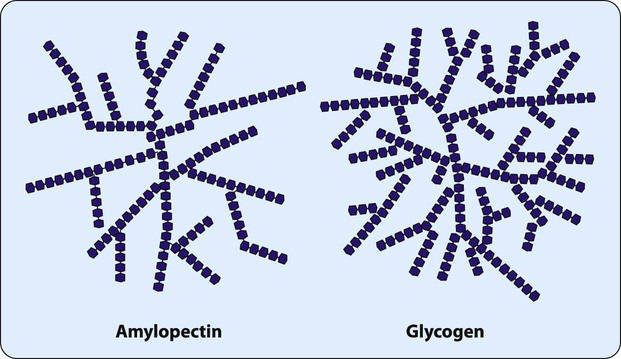
Many monosaccharides combine to form a polysaccharide. As shown in Table 2-2, polysaccharides can be characterized by the monosaccharide monomer present and overall function. One of the most important dietary polysaccharides is starch, the storage form of energy in plants. Starch is composed of two different polysaccharides (α-amylose and amylopectin). Figure 2-6 shows the linear structure of the polysaccharide α-amylose. Figure 2-7 shows the branched structure of the polysaccharide amylopectin. Important for the storage of energy in animals, glycogen also is a branched polysaccharide containing glucose monomers. The highly branched structure of glycogen allows its rapid degradation into glucose when energy is needed.
Table 2-2
Summary of common polysaccharides
| Name | Monomer | Biological Function |
| Amylose (in starch) | Glucose | Nutrient storage (plants) |
| Amylopectin (in starch) | Glucose | Nutrient storage (plants) |
| Glycogen | Glucose | Nutrient storage (animals) |
| Dextran | Glucose | Nutrient storage (yeast and bacteria) |
| Inulin | Fructose | Nutrient storage (plants) |
| Cellulose | Glucose | Structure in plants |
| Pectin | Galacturonic acid | Structural rigidity in plants and gelling agent in yogurt and jelly |
| Lignin | Coniferyl alcohol | Structural rigidity in plant cell walls |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses