Introduction
The use of a compound topical anesthetic (CTA) instead of an injection of a local anesthetic for placing miniscrew implants offers advantages to both the clinician and the patient. The purpose of this crossover, prospective, randomized clinical trial was to compare the clinical effectiveness of a CTA with that of a needle injection of local anesthetic for miniscrew placement.
Methods
Twenty-four orthodontic patients in a university clinic were recruited; they required bilateral buccal miniscrews for orthodontic anchorage. Eligibility criteria included healthy patients with no special needs; over 8 years of age and 25 pounds; not taking sulfonamides, monoamine oxidase inhibitors, tricyclic antidepressants, or phenothiazines; and not allergic to ester-type local anesthetics or any of the other materials used in the study. A computer generated a randomization list. The allocation was randomized by anesthetic protocol and side of the mouth, and was restricted to achieve balance by treatment and side of the mouth. No allocation concealment was applied. Associated with each randomized number was the subjects’ assignment into 1 of 4 groups divided by the side of first miniscrew placement and the type of anesthetic. Blinding was done only for data analysis because of clinical limitations. Each patient received a CTA on one side and an injection of anesthetic on the other before miniscrew placement in a crossover study design. The outcome was assessed by measuring pain levels with a 100-mm visual analog scale at 5 time points. Anesthetic failures occurred when the miniscrew could not be fully comfortably placed with a given anesthetic. Data were organized by visual analog scale time points, and descriptive statistics were calculated. A factorial repeated-measures analysis of variance was used to determine any differences.
Results
Twenty-seven patients were assessed for eligibility, and 24 agreed to participate in the study. Patients did not distinguish any differences in pain between the application of the CTA and the injection before or during anesthetic placement, but they experienced more pain with the CTA during miniscrew placement. The mean difference for the entire procedure between the 2 anesthesia types was 24.6 units, and the 95% confidence interval was 18.8 to 30.4, a statistically significant finding ( P = 0.0002). The CTA was still viewed as more painful 1 month after the procedures. Significantly more anesthetic failures occurred with the CTA (41.6%) than with the injection (0%). No serious harm was observed in any patient; when significant pain was observed with the CTA, a needle injection of local anesthetic was administered.
Conclusions
CTAs provided less predictable, often inadequate, and less comfortable anesthesia than an injection of a local anesthetic for managing patient discomfort during miniscrew placement in buccal sites.
Registration
This trial was not registered.
Protocol
The protocol was determined and approved by the research committee and institutional review board before the trial.
Funding
No external funding was used other than the donation of the miniscrews from Rocky Mountain Orthodontics, and no conflict of interest was declared.
Highlights
- •
Miniscrews were placed with compound topical and injected local anesthetics.
- •
Patients receiving the compound topical anesthetic had more pain during miniscrew placement.
- •
Compound topical anesthetics gave less predictable protection during buccal miniscrew placement.
Miniscrew implants, a type of orthodontic temporary anchorage device, are a simple yet effective means of increasing the efficiency and predictability of orthodontic treatment. Mah and Bergstrand advocated that orthodontists should place miniscrew implants (miniscrews) because of their better understanding of the biomechanical requirements and of the optimal placement of the miniscrews. However, a recent survey reported that only 55% of orthodontists were placing miniscrews themselves, and the reported reasons for referring it out were the invasiveness of the procedure and the associated pain and anxiety for the patient. Topical anesthesia, rather than needle injections of anesthetic, is a less invasive method of pain control that may decrease patient anxiety and simplify the anesthetic procedure for the orthodontist.
Although many clinical studies describe placing miniscrews using needle injections of local anesthetic, some authors have suggested that a compound topical anesthetic (CTA) could be used as the sole anesthetic for placing miniscrews. Shirck et al surveyed 61 orthodontists in private practice in the United States about their miniscrew use and found that 30.8% use only CTAs when placing miniscrews. There are several advantages of using a CTA for placing miniscrews, including patient comfort; simplicity of the procedure for the orthodontist; lack of tissue ballooning, which can obscure the miniscrew implant placement site; and patient feedback if the miniscrew is placed too close to the root structure.
Specific objectives and hypotheses
Since there are primarily only expert opinion reports in the literature, it is unclear whether a compound multidrug topical anesthetic is as clinically effective as a traditional needle injection of local anesthetic for pain control during miniscrew placement. The purpose of this study was to compare the clinical effectiveness of a compound multidrug topical anesthetic application with a traditional needle injection of local anesthetic in managing patient discomfort during miniscrew placement. The hypothesis was that a topical anesthetic provides the same level of pain control for miniscrew placement as does needle-injected local anesthetic.
Material and methods
Trial design
This was a cross-over, prospective, cross-arch randomized, controlled clinical trial with a 1:1 allocation ratio for anesthetic use.
Participants, eligibility criteria, and settings
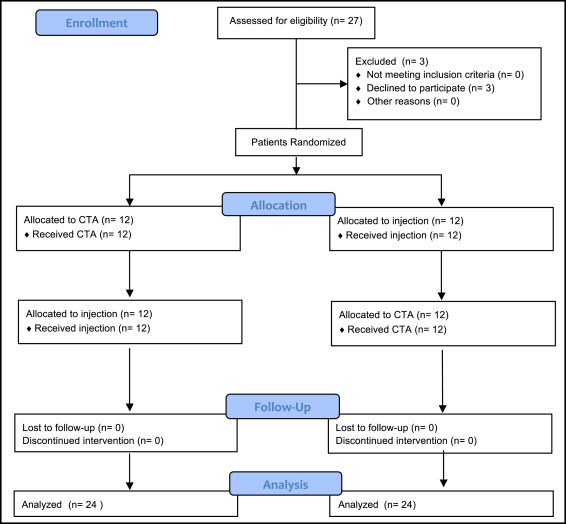
A total of 27 patients undergoing orthodontic treatment at the Department of Orthodontics clinic, School of Dental Medicine, at the University of Colorado were recruited as study participants. Only 24 of them agreed to participate in the study. Approval from the Colorado Multiple Institutional Board was obtained before patient recruitment (protocol number 10-0897). Consecutive orthodontic clinic patients meeting the following inclusion criteria were enrolled: (1) healthy, with no significant medical findings or special needs; and (2) requiring at least 2 miniscrew implants that were buccally located on opposite sides of the dental arch, placed in similar anteroposterior locations, and in the same dental arch. Exclusion criteria were patients (1) who were currently taking or had recently taken sulfonamides, monoamine oxidase inhibitors, tricyclic antidepressants, or phenothiazines; (2) with allergies to ester-type local anesthetics or any of the other materials used in the study; (3) with hypertension; (4) with past or current cardiac disease; (5) who use tobacco; (6) with bone metabolic disease; (7) who weighed less than 25 lbs or were less than 8 years old; (8) with same-day use of analgesics before the procedure; and (9) who were unable or unwilling to consent to the study.
Interventions
At the time of recruitment, each participant who met the inclusion and exclusion criteria was given a brief explanation of the study by 1 author (J.A.L.). Verbal and written consents to participate in the study were obtained from both the patient and the parent. Because only patients requiring 2 miniscrew implants were recruited for this study, the 2 types of anesthetics were compared in the same patient in a crossover, cross-arch study design. The 2 miniscrew placements, with the different anesthetics, were separated by at least 7 days to allow sufficient time for the patient to disassociate the 2 procedures. The placement sequence of the 2 anesthetic types and the side of the dental arch first treated were randomized between consecutive patients.
The CTA used in this study was a compounded mixture of 10% prilocaine, 10% lidocaine, 4% tetracaine, and 2% phenylepherine. The CTA was specifically compounded by Steven’s Pharmacy (Costa Mesa, Calif) based on the prescription provided by an author (R.E.H.). This specific mixture of topical anesthetics and phenylephrine, called PFG by Steven’s Pharmacy, was also recommended by other authors. A cotton tip applicator was used to administer the CTA for each procedure. In a pilot study, we found that the amount of CTA administered with 1 cotton tip application had a mean weight of 0.18 ± 0.04 g; this confirmed the pharmacy’s claim that a coated cotton tip application of the CTA was equal to approximately 0.125 to 0.25 g. Before CTA application, the soft tissue site for miniscrew placement was thoroughly dried with a 2 × 2-in piece of gauze. The CTA was applied on and around the attached gingiva and the alveolar mucosa of the miniscrew placement site with a cotton tip applicator and left in place for 2.5 minutes. The area was then wiped with gauze and rinsed thoroughly, as per the pharmacy’s recommendation.
The needle-injected anesthetic was 2% lidocaine hydrochloride with 1:100,000 epinephrine (Henry Schein, Melville, NY). Before the needle injection, the soft tissue site for miniscrew placement was dried with a 2 × 2-in piece of gauze. Then a cotton tip swab with 0.25 g of a single drug topical anesthetic, consisting of 20% benzocaine (Benzo-Jel; Henry Schein), was placed for 2 minutes to lessen the patient’s discomfort during the needle penetration. Approximately 0.45 mL of lidocaine, or a quarter of a carpule, was then injected as a buccal infiltration into the alveolar mucosa around the miniscrew placement site. A 0.45-mL dose of lidocaine provides an acceptable degree of anesthesia, while still allowing patient feedback in case the miniscrew contacts the periodontal ligament (PDL). All anesthetic expiration dates were checked before use to ensure efficacy. The miniscrews used in this trial were Dual-Top Miniscrews (Rocky Mountain Orthodontics, Denver, Colo) and were placed with a hand driver. All miniscrew implants had a 1.6-mm-diameter width and a 6-mm length for use in the anterior segments, and an 8-mm length for use in the posterior segments.
Outcomes (primary and secondary) and any changes after trial commencement
The outcomes measured in this trial were the patients’ comfort levels during miniscrew placement with the CTA and the needle-injected anesthetic. A visual analog scale (VAS) was used for measuring the pain levels; it is a common and proven method of measuring pain levels in patients. The participants were familiarized with the 100-mm VAS for pain measurement in a scripted presentation. They were asked to indicate their degree of discomfort (0 mm, no pain; 100 mm, worst pain imaginable) on the VAS survey form before receiving the anesthetic and before the miniscrew was placed (T0). This pretreatment VAS survey was used to determine the preoperative pain level and allow comparison of the consistency of the 2 baseline VAS scores from the same subject at 2 dates. One author (J.A.L) monitored the recording at T0 to ensure each subject’s full comprehension of the treatment protocols. The procedure began immediately after the T0 VAS scoring.
A VAS survey was taken immediately after each time point of the anesthetic protocols. The patient was asked to evaluate the pain felt during the anesthesia procedure only (T1) by completing the VAS survey immediately after anesthetic administration. The miniscrew was then placed by the patient’s orthodontic resident. Multiple residents, each well trained and experienced in miniscrew placement, placed the miniscrews during the trial; both miniscrews were placed by the same orthodontic resident in the same patient. Immediately after miniscrew placement, 2 postoperative VAS surveys were filled out (T2a and T2b). Each subject first evaluated the pain felt during only the miniscrew placement (T2a); on the second survey, the patient evaluated the overall pain experience for the entire procedure, combining the anesthesia and miniscrew procedures (T2b). The levels of discomfort (0 mm, no pain; 100 mm, worst pain imaginable) for each procedure were recorded on separate VAS surveys. Before each miniscrew was placed, the patients were told that if they felt greater discomfort than what they deemed moderate discomfort, they could be given more anesthetic delivered by injection. If at any time during either miniscrew procedure the patient felt a need for more anesthetic, the procedure was stopped and the subject completed both VAS surveys (T2a and T2b). This was counted as an anesthetic failure for that anesthetic group. A needle injection of lidocaine anesthetic was then given by infiltration until the subject was comfortable, and the procedure was completed. If the PDL was inadvertently contacted with a miniscrew, as evidenced by the patient’s response and the tactile sensation of the operator, that patient would be excused, and any data collected from that patient would be eliminated from the study. The patient could continue treatment but not as a study patient. No patient in the study experienced PDL pain during miniscrew placement.
The second miniscrew placement appointment occurred a minimum of 7 days after the first to allow the patients to disassociate any pain they might have experienced during the first procedure. A baseline VAS survey (T0) was again scored preoperatively, and each subject was given the other anesthetic from that used for the opposite side of the mouth. Immediately after the second anesthetic procedure, the patient completed another VAS (T1) survey to evaluate the pain felt during the anesthesia procedure only. Immediately after the second miniscrew placement, both follow-up VAS surveys were completed by the patient (T2a and T2b).
One month after the second miniscrew procedure, the patient evaluated the combined overall pain experienced during the first anesthetic and miniscrew procedure, and then the second anesthetic and miniscrew procedure on separate VAS surveys (T3). These surveys represented the patient’s recollection of the level of pain experienced while placing the miniscrews. The patients were reminded which anesthetic was used at each appointment. That completed the study, and each patient was given a $10 gift card.
Both anesthetic procedures were administered by 1 researcher (J.A.L.), experienced in both procedures, to eliminate confounding variables. No changes in the protocol were made to the trial after it started.
Sample size calculation
The sample size was estimated using a previous study’s standard deviation of 32 mm and a clinically significant difference of 20 mm on a 100-mm VAS at a power of 0.80 and an alpha of 0.05, resulting in 21 subjects needed per anesthetic type. Since each patient had one of each type of anesthetic, 21 patients were required for the study. A total of 27 patients were recruited for an expected attrition rate of less than 20%. Three patients declined to participate in the study.
Interim analyses and stopping guidelines
No interim analysis was performed during the study, and no guidelines were established for stopping the trial. However, as part of the protocol, the miniscrew placement procedure on a patient was stopped if he or she complained of pain.
Randomization (random number generation, allocation concealment, implementation)
The sequence of anesthetic placement and the side of the mouth were randomized. A randomization list was generated by computer. The allocation was randomized by the anesthetic protocol and the side of the mouth, and was restricted to achieve balance by treatment and side of the mouth. No allocation concealment was applied ( Fig 1 ).
Blinding
Blinding of the patient and the clinician was not possible during the trial because of the nature of the trial. Blinding occurred only during the data analysis. The measurement of the VAS surveys was performed by 1 author (J.A.L.), who, during data analysis, was blinded to the anesthetic type and the sequence used for each procedure by the randomized number assigned to each patient.
Statistical analysis (primary and secondary outcomes, subgroup analyses)
The data were organized by the VAS survey time points, and descriptive statistics were calculated (means and standard deviations) for the topical and injection modes of anesthesia delivery. We observed a numeric difference in VAS measurements and, therefore, performed a factorial repeated measures analysis of variance (ANOVA) for this data set (SPSS version 19; IBM, Armonk, NY). This test analyzed differences between the anesthetic types (topical vs injection) and also within the participants’ variables (T0, T1, and T2a) with repeated measurements. This allowed us to determine the main effects of the treatment types, time passage, and also their interactions. The alpha level for all statistical tests was set at P ≤ 0.05. Age and sex vs VAS score at each time point were graphically evaluated, and the linear correlation coefficients were calculated to investigate the effects of age or sex on the VAS measurements. Additionally, factors such as age, sex, T0 (baseline VAS), and T1 (anesthetic VAS) were evaluated for significance between the failures and the nonfailures to assess their usefulness as predictors of success or failure of the CTA procedure using t tests in each group.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


