Introduction
The aim of this study was to investigate the microbiologic and immunologic factors related to orthodontic treatment-induced gingival enlargement (GE).
Methods
Our study included 12 patients with GE undergoing fixed orthodontic treatment and 12 periodontally healthy controls. At baseline, periodontal variables, subgingival plaque samples, and gingival crevicular fluid (GCF) samples were taken from 2 preselected sites in both the GE and the control groups. The levels of Porphyromonas gingivalis , Aggregatibacter actinomycetemcomitans , Prevotella intermedia , Treponema denticola and Tannerella forsythia were determined by real-time polymerase chain reaction. GCF interleukin (IL)-1β and transforming growth factor-beta 1 (TGF-β1) were detected by enzyme-linked immunosorbent assay (Invitrogen, Camarillo, Calif). Periodontal therapy was given to the GE group, and all parameters were reassessed after 4 weeks.
Results
At baseline, the GE group showed higher prevalences of the 5 periodontal pathogens than did the control group ( P <0.05). IL-1β and TGF-β1 levels at the GE sites were also significantly higher than those at the control sites ( P <0.05). Four weeks after periodontal therapy, the GE group showed significant improvements in the clinical parameters associated with significant reductions of P gingivalis , A actinomycetemcomitans , and T denticola . The levels of IL-1β decreased significantly compared with the baseline ( P <0.05), whereas there was no significant change in TGF-β1 levels ( P >0.05).
Conclusions
Periodontal pathogens might have a relationship with the initiation and development of orthodontic treatment-induced GE. Inflammatory cytokines (IL-1β and TGF-β1) can also be considered as contributing factors.
Gingival enlargement (GE) is a common complication of orthodontic treatment ; it can begin within 1 to 2 months after placement of appliances. The presence of fixed appliances influences plaque accumulation and the colonization of important periodontopathic bacteria. When gingival tissues are enlarged, the tooth surfaces become difficult to access, inhibiting good oral hygiene and resulting in more inflammation and bleeding. Since periodontal pathosis at a young age could eventually impose a burden over the long term, the interruption of orthodontic treatment is often advised when GE is diagnosed. Thus, temporary removal of irritating factors, such as attachments and appliances, periodontal debridement, chlorhexidine prophylaxis, and surgical intervention such as flap surgery can be used in some patients to restore the contour of the enlarged gingival tissues and facilitate adequate oral hygiene during subsequent orthodontic treatment.
To date, only a few studies have focused attention on the pathogenesis and management of orthodontic treatment-induced GE. Kloehn and Pfeifer indicated that mechanical irritation by bands, chemical irritation by cements used for banding, food impaction, and less efficient oral-hygiene maintenance are etiologic factors of orthodontic treatment-induced GE. Alexander characterized this phenomenon as an inflammatory response to the plaque microbiota and related by-products, because enlarged gingival tissues can influence the subgingival ecosystem by creating an appropriate anaerobic environment, leading to a shift in the composition of the microflora. In a recent study, Gursoy et al suggested that a continuing low dose of nickel released to the epithelium was the initiating factor in gingival overgrowth from orthodontic treatment.
The initiation and development of periodontal disease depend on a dynamic equilibrium between the microbial challenge and the host’s immune-inflammatory responses. Periodontal pathogens express multiple virulence factors, such as lipopolysaccharide and peptidoglycan, stimulating host cells to release several kinds of inflammatory cytokines. There is substantial evidence that cellular and molecular changes in the gingival extracellular matrix leading to GE are regulated via various cytokines and growth factors, including interleukin (IL)-1β, IL-6, and transforming growth factor-beta 1 (TGF-β1). The aim of our study was to evaluate (1) the microbiologic and immunologic factors (IL-1β and TGF-β1) related to orthodontic treatment-induced GE and (2) the clinical, microbiologic, and immunologic effects of periodontal therapy on orthodontic treatment-induced GE.
Material and methods
Twenty-four adolescent subjects, 15 boys and 9 girls, aged 12 to 18 years, participated in this study; the GE group consisted of 12 subjects undergoing fixed orthodontic therapy for at least 6 months (mean duration, 9.4 ± 2.5 months) at the Department of Stomatology, Zhongshan Hospital, Fudan University, Shanghai, China. Each patient had at least 2 papillae covering one third to two thirds of the clinical crown, diagnosed as orthodontic treatment-induced GE. Before orthodontic treatment, all subjects had no clinical signs of gingivitis or periodontitis and received oral-hygiene instructions by the same clinician (Y.G.). The control group consisted of 12 periodontally healthy subjects, matched for age and sex.
All subjects fulfilled the following criteria for participation in this study: (1) good general health with no history of systemic disease, (2) no alveolar bone loss visible on x-rays, (3) no periodontal treatment within the last 6 months, (4) no antibiotic therapy in the last 6 months, (5) no medication known to cause GE, including phenytoin, cyclosporine, nifedipine, verapamile, diltiazen, felodipine, or nitredipine. Informed consent was obtained from the patients or their parents before the study. The research protocol was approved by the ethics committee of Fudan University.
The clinical parameters were taken at baseline by the same doctor. In the GE group, measurements were made at 2 preselected GE sites in the anterior mandibular region where orthodontic patients normally exhibit severe GE. Two periodontally healthy sites from the same region were also measured in the control group.
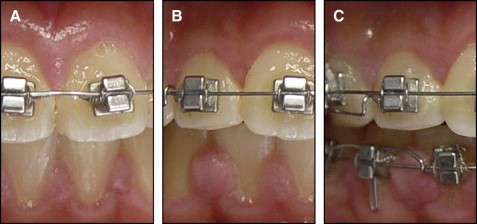
The degree of GE was assessed in 4 categories on the basis of the hyperplastic index (HI) as developed by Angelopoulos and Goaz and further modified by Pernu et al : 0, no gingival overgrowth; 1, mild overgrowth, blunting of the marginal gingiva; 2, moderate overgrowth, extending to the middle of the tooth crown; and 3, severe overgrowth, covering two thirds of the tooth crown or affecting the whole of the attached gingival ( Fig 1 ). Probing depth (PD) in millimeters was recorded by using a periodontal probe (PCP UNC15, Hu-Friedy, Chicago, Ill) at 3 sites around the interdental area (mesiobuccal, midbuccal, and distobuccal sites). The plaque index (PI) was also evaluated according to the method of Silness and Loe, and the papillary bleeding index (PBI) was evaluated according to the method of Saxer and Muhlemann.
Subgingival plaque and gingival crevicular fluid (GCF) samples were also collected from the same sites after measuring the PI and before measuring the PBI and the PD in both groups. After careful removal of all supragingival plaque, the areas were washed with a water spray, isolated with cotton rolls, and gently dried (30 seconds). A sterile paper point (#30) was placed into the bottom of the periodontal pocket for 30 seconds. Points with marks of blood were discarded. The paper points were placed in sterile polypropylene tubes containing 1.5 mL of phosphate-buffered solution.
The teeth were washed again; the area was isolated and then gently dried. GCF was collected with paper strips (Whatman International, Maidstone, United Kingdom) placed into the gingival crevice and maintained for 30 seconds. Strips with marks of blood were discarded. The paper strips were placed in sterile polypropylene tubes. One examiner performed all microbial and GCF sampling. All samples (subgingival plaque and GCF) were stored at –20°C.
After recording the parameters, the archwires and brackets at the GE sites were temporarily removed to allow maintenance of adequate plaque control. Then, 1 section of full-mouth periodontal debridement was performed with an ultrasonic scaler with a time limit of 45 minutes. Chlorhexidine prophylaxis (0.12% chlorhexidine gluconate; South China Pharmaceutical Co, Ltd, Shenzhen, China) was also administered twice a day for 2 weeks after the periodontal treatment. Oral-hygiene instructions were reinforced again.
Four weeks after the periodontal treatment, the clinical parameters and the collected samples were reassessed at the same sites in the GE group.
The real-time polymerase chain reaction (PCR) method used in this study was based on the amplification of variable regions of the 16S rRNA genes of Porphyromonas gingivalis (Pg), Aggregatibacter actinomycetemcomitans (Aa), Prevotella intermedia (Pi), Treponema denticola (Td), and T annerella forsythia (Tf). Briefly, species-specific primers were selected by using software (Primer Premier version 5.0; Premier Biosoft International, Palo Alto, Calif) based on the published 16S rRNA sequences. The primers used in this study are listed in Table I .
| Primer | Sequence (5′–3′) | Size of amplicon (bp) | Accession numbers |
|---|---|---|---|
| Pg | F1:GGAATAACGGGCGATACGA | 155 | X73964 |
| F2:CACCGCTGACTTACCGAACA | |||
| Aa | F1:ATTGGGCATAAAGGGCATCT | 204 | X90833 |
| F2:TTCGCACATCAGCGTCAGTA | |||
| Pi | F1:GCCTAATACCCGATGTTGTCC | 237 | L16468 |
| F2:ACTTGGCTGGTTCAGACTTCC | |||
| Td | F1:CTGAGGACTCTGGCGGAACT | 228 | D85438 |
| F2:ACCGTGCTGATGTGTGCGATTA | |||
| Tf | F1:AGAGCCTGAACCGGCCAAGT | 208 | L16495 |
| F2:ACAGCCCCACCTACGCACC |
Bacterial DNA was extracted as described previously. A primer concentration of 0.25 μmol/L was ultimately used for the 5 species. Real-time PCR reaction was carried out by using a Mastercycler system (Eppendorf, Wesseling-Berzdorf, Germany) with SYBR Green Mix (Ruicheng Biotech, Shanghai, China). Samples were assayed in duplicate in 25-μL reaction mixtures containing 2 μL of template DNA, 0.5 μL of forward primer and reverse primer, and 12.5 μL of SYBR Green Mix. The cycling conditions used were as follows: 95°C for 15 minutes, 95°C for 30 seconds, 53°C for 30 seconds, 72°C for 30 seconds, and 40 cycles for Pg and Tf; and 95°C for 15 minutes, 95°C for 30 seconds, 54°C for 30 seconds, 72°C for 30 seconds, and 40 cycles for Aa, Pi, and Td. Melting peaks were used to determine the specificity of the PCR.
Absolute quantification of target bacteria in the subgingival samples was performed by using Pg (ATCC 33277), Aa (Y4), Pi (ATCC 23256), Td (ATCC 33520), and Tf (ATCC 43037) as controls. Standard curves were established with the controls that could be used to convert cycle threshold values into the number of bacterial cells by using controls with known amounts of bacterial-specific DNA. The level of detection was set at 10 3 bacteria per subgingival plaque sample for real-time PCR. The determination of the DNA content in the controls was based on the genome size of each bacteria and the mean weight of 1 nucleotide pair.
GCF IL-1β and TGF-β1 levels were analyzed with ELISA by using the relevant ELISA kit (enzyme-linked immunosorbent assay; Invitrogen, Camarillo, Calif) according to the manufacturer’s instructions. GCF samples were eluted from the strips by placing them in 250 μL of phosphate-buffered solution. The dilution was considered to calculate the concentration of each GCF substance. The results were expressed as picograms per site.
Statistical analysis
The data were analyzed by using the SPSS statistical package (version 13.0, SPSS, Chicago, Ill). Individual sites were analyzed for clinical, subgingival microbiologic, and immunologic parameters.
The Mann-Whitney U test and the Wilcoxon signed rank test were used to detect significant intragroup and intergroup differences with regard to clinical periodontal parameters and GCF cytokine levels at the test sites. For the microbiologic parameters, the frequencies of periodontal pathogens were compared by using the Fisher exact test. Logarithmic transformations were performed for the amounts of bacteria to improve normality; the amounts of bacteria were compared with the Student t test. For all tests used, values of P <0.05 were considered statistically significant.
Results
At baseline, all evaluated clinical parameters (including PI, PBI, PD, and HI) of the test sites in the GE group were significantly higher than those in the control group ( P <0.001). Four weeks after periodontal treatment, these clinical parameters decreased significantly compared with those at baseline ( P <0.001).There was no significant difference for PI when compared with the control group ( Table II ). At baseline, 96% of the test sites in the GE group scored 2, and 4% scored 3 for HI. After 4 weeks, the HI of the test sites decreased; only 21% of the sites scored 2, 46% of the sites scored 1, and 33% of the sites scored 0.
| Periodontal index | GE group | Control group | P value | |||
|---|---|---|---|---|---|---|
| Baseline | 4 weeks | Baseline vs control | Baseline vs 4 weeks | 4 weeks vs control | ||
| PI | 1.58 ± 0.65 | 0.29 ± 0.46 | 0.25 ± 0.44 | <0.001 ∗ | <0.001 ∗ | 0.748 |
| PBI | 1.54 ± 0.78 | 0.42 ± 0.58 | 0.08 ± 0.28 | <0.001 ∗ | <0.001 ∗ | 0.016 ∗ |
| PD (mm) | 3.18 ± 0.44 | 2.42 ± 0.56 | 1.86 ± 0.28 | <0.001 ∗ | <0.001 ∗ | <0.001 ∗ |
| HI | 2.04 ± 0.20 | 0.88 ± 0.74 | 0.13 ± 0.34 | <0.001 ∗ | <0.001 ∗ | <0.001 ∗ |
∗ Significant differences (Mann-Whitney U test or Wilcoxon signed rank test).
Overall, 10 of 12 patients in the GE group showed marked improvements in gingival condition 4 weeks after periodontal therapy. However, 2 patients had severe GE with moderate reductions in PI and PBI at the test sites (data not shown). Therefore, these 2 patients had surgical gingivectomy to restore the physiologic gingival contour and to facilitate good oral-hygiene maintenance during the subsequent orthodontic treatments.
At baseline, the frequencies of Pg, Aa, Pi, Tf, and Td in the GE group were significantly higher compared with those in the control group ( P <0.05). Four weeks after periodontal treatment, the frequencies of Pg, Aa, and Td decreased significantly ( P <0.05). Compared with the control group, no significant differences were found for the 5 bacteria ( P >0.05) ( Table III ).



