Fig. 10.1
Spontaneous numbness and pain in the facial region: (a) a 36-year-old male with right facial pain and swelling (arrow) and numbness of the right upper lip; (b) panorex shows lesion of right maxilla and sinus (white arrows). Biopsy revealed transitional cell carcinoma. Microscopic examination showed tumor invasion of the right infraorbital nerve. (c) A 41-year-old female with right mandibular pain and numbness of right lower lip and chin (outlined); (d) panorex reveals lytic lesion (white arrows) involving right mandible and IAN. Biopsy showed metastatic adenocarcinoma, due to primary tumor in uterus
The date of the incident and/or onset of sensory changes is pertinent because there is a timetable for the pathophysiologic response of a peripheral nerve to injury (Wallerian degeneration) [7, 61]. Progressively, the axons distal to the injury location undergo necrosis and phagocytosis. As this process is completed, repair is begun by outgrowth of axonal sprouts from the proximal nerve stump. If the distal nerve superstructure is not recannulated by new axons within a reasonable period of time, it is replaced by scar tissue and becomes incapable of repair, either spontaneously or by surgical intervention. Although there is some uncertainty regarding the timing of surgical repair of nerve injuries [64], it is generally accepted that there is a window of opportunity of about 6 months from the time of injury when surgical repair of an injured nerve provides the best chance of improvement or restoration of sensory function [2–5]. After that, the chance of successful outcome of nerve repair decreases with each passing month until a critical mass of distal nerve tissue is replaced by scar tissue that lacks the potential for restoration of nerve function; additionally, there is ganglion cell death in the trigeminal ganglion that decreases the total percentage of possible sensory recovery. In humans, this time has been estimated at 12 months or longer, depending on the age and general health of the patient and other factors not yet fully understood [42]. In any case, it behooves the clinician who initially attends the patient with a sensory nerve injury to note the date of injury so that surgical intervention that might be indicated for non-resolving sensory dysfunction can be done within a favorable time frame.
Numbness or pain may not begin concomitantly with the incident or operation associated with the nerve injury. For example, seepage of root canal medicaments from the tooth apex following over-instrumentation during root canal preparation may take one or more days to reach the adjacent inferior alveolar canal (IAC) and cause a chemical burn of the IAN. Similarly, after bone preparation for insertion of dental implants, edema secondary to heat generated by the drill may develop slowly within the IAN, producing delayed compression of the nerve with the onset of lower lip numbness and/or pain not noticed by the patient for up to 24 h after the procedure. Also, if the IAN is not directly injured, but the bony wall of the IAC is disrupted during elevation and removal of a mandibular third molar, or during any other procedure (e.g., mandibular fracture or mandibular osteotomy), excessive bone may be regenerated during the healing process [9]. Thus, the IAC diameter is narrowed, and delayed compression of the IAN occurs one to several months later with the onset of symptoms at that time. Such instances help to explain why, although most sensory nerve injuries result in immediate onset of symptoms, in some patients sensory dysfunction might occur later and render the association between cause and effect somewhat obscure.
The progression of sensory symptoms is significant because, over an interval of days, weeks, or months after the injury, the patient might show improvement or deterioration of sensory function or undergo no change [18]. The patient is seen at regular intervals (i.e., every 2–4 weeks) for repeated evaluations to ascertain any evolution of sensory status. In patients who are improving, an expectant course can be taken; serial examinations are repeated as long as they continue to show documented subjective and objective improvement at each subsequent visit. A patient who fails to show improvement of neurosensory status from one evaluation to the next (especially beyond 3 months following nerve injury) will generally not resume improvement at some future date. This patient is assumed to have reached a plateau or end point. If his or her sensory status is judged to be unacceptable, a decision regarding surgical intervention should be considered at that time rather than continuing to follow the patient further in the vain hope that further improvement will occur in the future. Whether or not a patient is improving is based not only upon subjective information (the patient’s history) but also upon objective evidence (the examination, see below). In the course of recovery from a sensory nerve injury, new symptoms may appear. Most commonly, numbness is the patient’s initial complaint. Although there may be pain at that time as well, it often develops days or weeks after the injury, and it may increase in frequency, duration, and intensity, be episodic initially and then become constant, and be spontaneous or associated with various orofacial maneuvers or daily activities.
Aside from the unpleasant sensory symptoms, many patients experience interference with normal daily activities or functions (see Appendix 10.A.1, item #7). Chewing food, drinking liquids, toothbrushing, face washing, shaving, applying lipstick and makeup, and speaking are examples of common acts that are performed almost without thinking in the person with normal orofacial sensory and motor function. Loss of sensory input adversely affects the coordination of the motor component of any activity. Therefore, accidental lip or cheek biting while chewing food, dribbling of liquids while drinking, difficulty with toothbrushing or application of lipstick, and alterations of speech are common complaints of the patient with a peripheral trigeminal nerve injury and should be duly noted [27]. In some patients, interference with speech or the ability to play wind musical instruments may impact on their capacity to earn a living. Referral to a speech pathologist or other performing consultant may be indicated in order to properly document a loss of function and arrange for appropriate corrective therapy, if indicated.
Although not a primary complaint, the patient with a lingual nerve injury is often aware of alterations of taste sensation (parageusia, dysgeusia) that may be characterized as a general lessening or loss of taste, loss of one or more specific taste senses (sweet, sour, salty, bitter), or a foul or unpleasant taste (e.g., metallic, rotten, foul, rancid). The patient should be counseled that taste sensation may improve along with spontaneous improvement in general sensory function of the lingual nerve (LN) or as a result of the microsurgical repair of the LN [53]. However, it is further explained that taste sensation is transmitted by the chorda tympani fibers from the facial nerve (FN7) that travel with the lingual nerve but which send their impulses to the nucleus of the FN7 and have a potential for healing and recovery of function that is not as great as that of the LN. Therefore, recovery of taste may or may not occur to the same extent as that of the general sensory function of the LN, although some patients report normal or near-normal taste sensation after LN repair [6, 29, 53, 69].
As important as is the history in the evaluation of a patient’s complaint, it has been shown that neurosensory problems can be over-reported by some patients [14]. Therefore, it behooves the clinician to always complete a comprehensive neurosensory examination of the patient regardless of the alleged severity of the subjective symptoms.
10.3 Equipment
The well-equipped practitioner’s office will already contain the supplies and instruments required for examination of the nerve injury patient. Sterile gloves, mouth mirror, tongue blades, cotton swabs, calipers, local anesthetic needles (27 gauge), anesthetic cartridges, and local anesthetic syringe are the basic armamentarium used for nerve testing (Fig. 10.2). A pulp tester (vitalometer) is sometimes used as method of assessing response to pain when evaluating an IAN injury (Fig. 10.3). An algometer can be another way to assess pain response. Thermal discs are utilized by some clinicians to test sensory response to temperature change [17]. Semmes-Weinstein monofilaments [63] provide a more accurate and reproducible measure of contact detection (static light touch), although the use of a cotton swab as demonstrated below is adequate in the typical clinical situation.

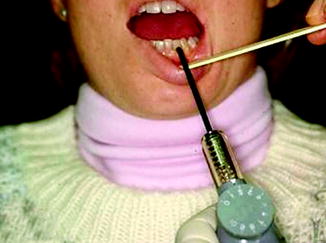

Fig. 10.2
Basic equipment for NST includes (left to right) syringe, local anesthetic cartridges, calipers, 27-gauge needle, tongue blade, cotton swabs, mouth mirror, and examination gloves

Fig. 10.3
A pulp tester can be used to assess pain response of the lower teeth in a patient with an IAN injury
10.4 Head, Neck, and Oral Examination
A regional examination is completed on all patients including the head, eyes, ears, nose, face, temporomandibular joints, neck, oral cavity, pharynx, and neck. Specific components of the screening evaluation for the nerve injury patient are shown in Fig. 10.4. Following recording of the patient’s vital signs, the next step is inspection. If the patient is acutely injured, the examiner looks for evidence of maxillofacial trauma (missile wound, laceration, facial bone fracture, abrasion, or contusion). A nerve injury (transection, avulsion, partial tear, compression, or crushing) may be able to be directly visualized through an open wound or laceration [2]. In other patients, the examiner searches for signs of recent or past injury or surgery (e.g., sutured or healing incisions, scars) or neurotrophic changes of the skin (edema, erythema, ulcerations, hypohidrosis, loss of hair, hypokeratosis) that may develop following sensory loss in that area. The patient with long-standing sensory dysfunction may repeatedly traumatize insensate soft tissues, producing factitious (self-induced) injury (Fig. 10.5). In the neck, scars from previous injury or surgical incisions when stimulated by repeated gentle stroking with the examining finger or a cotton swab may respond with symptoms and signs of sympathetic nervous system hyperactivity (hyperesthesia, sweating, blanching, flushing, skin temperature changes) in the cutaneous area supplied by the injured nerve. Such findings may be diagnostic of sympathetic-mediated pain (SMP; also known as reflex sympathetic dystrophy or complex regional pain syndrome [26]).
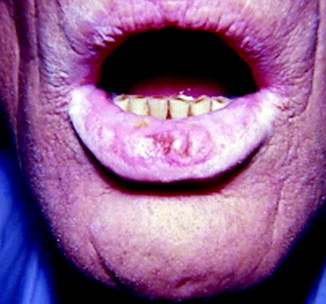

Fig. 10.4
The initial part of the NST includes inspection, palpation, and percussion of the head, neck, and oral regions. Positive findings in this screening process may lead the clinician to the location of the nerve injury and provide important information about its severity

Fig. 10.5
A 62-year-old fisherman with loss of sensation in lower lip and chin from injuries sustained from chronic lower lip biting for 20 years. The central portion of the lower lip, initially thought to show the results of self-induced injury, on biopsy was found to be squamous cell carcinoma, while the rest of the lower lip showed precancerous dysplastic changes
Palpation or percussion is done directly over the mandibular retromolar area or the medial surface of the mandible adjacent to the third molar tooth (for the LN), over the mental foramen either on the skin surface or intraorally between the mandibular premolar teeth (for the MN), beneath the inferior orbital rim on the skin or transorally superior to the maxillary premolar teeth (for the IFN), and at the midpoint of the eyebrow (for the supraorbital nerve (SON)). One of three possible responses, each called a trigger, may be induced in the presence of a nerve injury (Fig. 10.6). First, a painful sensation (often characterized by the patient as an “electric shock”) is induced and is limited to the area of applied stimulation. Second, this painful sensation may radiate from the area of stimulation and proceed distally into the area supplied by the affected nerve (e.g., palpation of the lingual nerve causes ipsilateral painful sensations in the tongue and floor of mouth). Third, nonpainful responses (tingling, crawling, itching) radiate from the area of nerve palpation. In some patients, palpation or percussion over the injured nerve induces no trigger response. Subsequent direct observations of the injured nerve during microsurgical repair usually confirm that the trigger area denotes the site of nerve injury [70]. A painful response without radiation frequently indicates a complete nerve severance with a proximal stump neuroma as the source of the pain. On other hand, a painful response, or a nonpainful response with radiation, in some patients is a sign of partial nerve transection or a neuroma-in-continuity. This sign has been referred to as the Tinel’s sign, and it may indicate regenerating nerve fibers present in the area of palpation, or it may indicate the presence of a neuroma. In other patients with complete nerve severance, there are distally radiating sensations from the trigger area which probably represent phantom pain [33]. Occasionally, a patient with a significant nerve injury fails to give a trigger response to stimulation over the injury site. Therefore, a trigger response should be considered indirect evidence of a significant nerve injury, whereas a lack of response does not rule out the presence of injury.
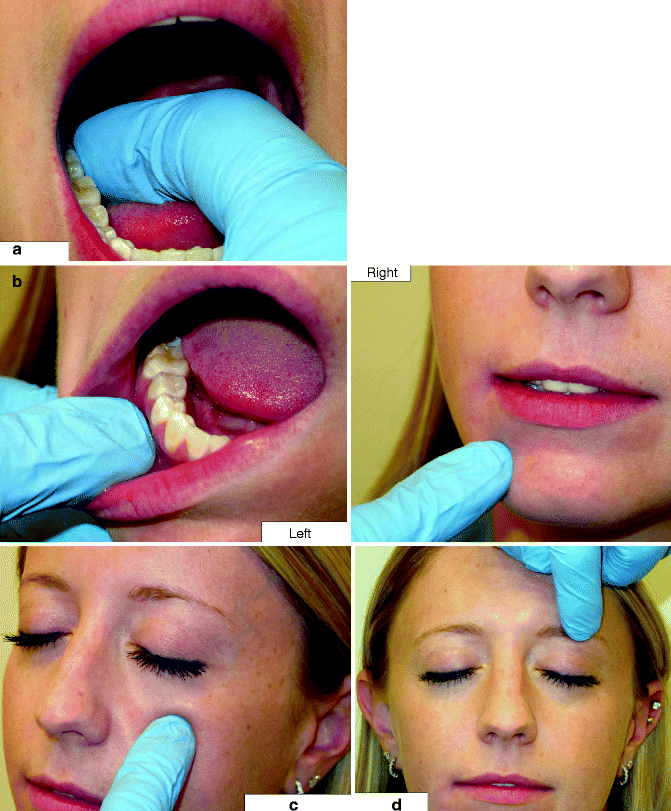

Fig. 10.6
Palpation is done directly over a nerve contained in soft tissue to check for a trigger response: (a) right LN is palpated on the lingual aspect of the mandibular third molar area; (b) the right mental nerve is palpated either intraorally in the mandibular buccal vestibule (left) or on the face (right); (c) the left infraorbital nerve is palpated on the face beneath the inferior orbital rim, but it can be accessed intraorally as well; (d) the left supraorbital nerve is felt as it exits the orbit just superior to the superior orbital rim
Percussion of the mandibular teeth may invoke tingling or unpleasant sensations that may or may not radiate from the teeth to the lower lip or chin. Palpation, percussion, or gentle stroking of the lower lip or chin may also cause sensations that radiate to the lower teeth. The significance of these findings in relation to the extent or nature of the injury to the IAN is not well understood [24, 25, 41, 66]. The appearance of the nerve at surgery does not always correlate well with the extent of injury implied by results of the clinical examination; examination and neurosensory testing (see below) are less accurate at predicting the extent of IAN injury than that of LN injury [70].
The evaluation of taste sensation requires special equipment (see below), it is a technically demanding endeavor [68], and the results may be difficult to interpret [28]. Most patients who have sustained an LN injury are primarily concerned with lost, altered, or painful general tongue sensation and the sequelae of accidental tongue biting, difficulty chewing food, painful toothbrushing (if there is a trigger area), effects on speech, and interference with the playing of wind musical instruments. Whether or not the patient has altered taste sensation seldom, if ever, influences the surgeon’s decision regarding surgical or other treatment for the injured LN [3, 53]. Therefore, taste testing is not usually included in the routine evaluation of the patient with a peripheral trigeminal nerve injury. In the patient who develops a taste aberration in the absence of known peripheral nerve injury, taste testing may be helpful in documenting whether or not there is an anatomical cause for actual loss of taste function (which might represent a symptom and sign of a brain tumor, for instance) rather than its being due to a side effect of medication (e.g., chlorothiazide diuretics) or to a strictly psychological aberration. The taste buds in the anterior two-thirds of the tongue receive special sensory supply from the chorda tympani fibers that originate in the nucleus of the FN7 and join the LN peripherally before its course to the tongue. Taste buds in the posterior one-third of the tongue are supplied by the glossopharyngeal nerve (GP9). Therefore, application of substances must be carefully confined to one or the other segments of the tongue, and crossover of the substances to the contralateral side must be prevented to allow valid interpretation of the results. Further complicating this special sense is that taste is greatly influenced by the sense of smell (note the common loss of taste sensation during an upper respiratory affliction such as the common cold or rhinitis from allergies). During taste testing, olfaction must be blocked, or nonaromatic substances must be used. Some patients have reported no change in taste sensation in instances of documented LN anesthesia [53]. Clinical testing has shown a “remarkable difference” between a patient’s stated impression of his taste perception and his true ability to taste specific substances based on testing [29]. Some patients may not even be aware of significant deficits in taste perception [8]. The ability of the chorda tympani nerve nuclei to regenerate after severance of peripheral axons has been shown to be highly unpredictable and sometimes to a lesser degree than the general sensory nerve nuclei of the trigeminal nerve [23]. Psychological factors not presently known or understood may influence a patient’s perception of taste, whether or not there has been an injury to one of the nerves carrying special sense impulses from the taste buds [52].
If the clinician wishes to evaluate taste sensation, the examination may include either regional testing or whole mouth testing [21]. Regional testing evaluates selected groups of taste buds (i.e., those on the anterior two-thirds or those on the posterior one-third of the tongue) and allows the clinician to differentiate between the special sensory input of the LN and the GP9 [28]. Therefore, regional testing is more valuable when one desires to measure the function of a specific nerve with regards to taste sensation [44]. Whole mouth testing gives a more global and nonspecific overview of the integrity of taste rather than focusing on the specific innervation of selected groups of taste buds. The sense of taste conducted from the taste buds supplied by the chorda tympani branch of the facial nerve via the LN can be tested by applying sweet (sucrose), sour (citric acid), salt (saline), and bitter (quinine) substances to the anterior two-thirds of the tongue [8]. The substances are applied via an enclosed “surface chamber” to confine them to an isolated and discrete area of the tongue [68]. The patient’s eyes are closed and the nares are occluded during the taste applications. The patient is requested to report whether they feel the application of the test substance on the tongue and to identify the specific taste. Findings are graded on a 0–2 scale (2 = patient feels application and correctly identifies its taste as sweet, sour, salt, or bitter; 1 = patient feels application, but has no taste identification; 0 = patient does not feel application and has no taste identification).
10.5 Neurosensory Testing
Neurosensory testing (NST) includes a group of standardized clinical diagnostic maneuvers designed to evaluate general sensory function in as unbiased a manner as possible. Such testing, although the methods have been characterized as being subjective in that they are influenced by the patient’s level of cooperation and interpretation [65], is reproducible on serial repetition on the same patient by the same or other examiners. The patient who is malingering, is considering a legal action against the practitioner who performed a procedure thought to be responsible for a nerve injury, or is attempting to embellish an application for worker’s compensation for a job-related injury may be a challenge for the clinician who strives to gain an accurate assessment of the level of sensory dysfunction. In some instances, the patient may seek to exaggerate responses to NST (as well as overestimate the severity of symptoms in the history). A patient who complains of symptoms or functional impairment in distinct disproportion to the results of the clinical examination should arouse suspicion that there is a “hidden agenda” involved with attempts to manipulate the results of the neurosensory testing. By randomizing the order, type, or location of stimulus application (or in some instances not applying the stimulus at all) and carefully observing the patient’s nonverbal responses (e.g., promptness or lack of response or withdrawal from a stimulus) or “body language” (disinterested facial expression, grimacing or sneering, failure to make eye contact during the evaluation, nervous hand mannerisms, excessive facial sweating, facial flushing or pallor), the astute examiner may be able to recognize inappropriate behavior and prevent patient attempts to distort or misrepresent the results.
The material presented below is a summary of methods used by clinicians experienced in the field of peripheral nerve injuries [16, 22, 36, 43, 48, 49, 67]. The rationale for their use and the validity of their results have been established in various studies [19, 70].
During the neurosensory examination, the patient is seated comfortably in a quiet room, and most maneuvers are performed with the patient’s eyes closed. When the patient’s lips are being tested, they should be separated so that pressure or vibration of applied stimuli is not transferred from the stimulated lip to the opposite lip. The specific tests and responses are described in detail to the patient so that he understands and is able to make the appropriate responses during NST. The examiner explains each step beforehand with reassurance that the stimulus will be applied gently and with due concern for any areas of pain or hypersensitivity that were described in the patient’s history or elicited in the general head, neck, and oral examination. The contralateral normal side is always tested first to determine the patient’s normal “control” responses in order to establish a baseline for examination of the abnormal side.
The NST begins by determining the area of altered sensation with the marching needle technique. A 27-gauge local anesthetic needle is advanced from a normal area adjacent to the area of sensory dysfunction indicated by the patient’s history. The needle contacts the surface mucosa or skin lightly at 1–2 mm intervals until the patient indicates (by raising the ipsilateral hand) the location where the sensation of the needlepoint begins to change. This process is repeated until the border of the entire area of altered sensation is determined. Within this area when the injured nerve has lost all ability to transmit impulses, there will be an intermediate zone adjacent to the border where there is a decrease in the appreciation of the stimulus (hypoesthesia in which sharp becomes “dull,” but contact is still perceived by the patient) [11]. This is probably due to crossover sensory fibers from an adjacent or contralateral nerve [20]. Further into the affected area (usually within a few millimeters), the patient fails to feel the stimulus at all (anesthesia). If this area is on the skin, it is indicated with a colored erasable marking pen. The markings can later be easily removed with alcohol or orange solvent (Fig. 10.7). The NST then begins, first, on the contralateral normal side (e.g., the right lower lip to establish normal responses for that patient) and then on the ipsilateral side (the left lower lip with altered sensation) to ascertain the level of abnormal responses. In a patient with bilateral nerve injuries, an adjacent normal area is chosen for control responses (e.g., for bilateral IAN injuries, the vermilion border of the normal upper lip for comparison with the abnormal lower lip; for bilateral IFN injuries, the normal lower lip vermilion border for comparison with the abnormal upper lip; for bilateral LN injuries, the normal lower labial mucosa for comparison with bilateral lingual gingiva and tongue numbness).
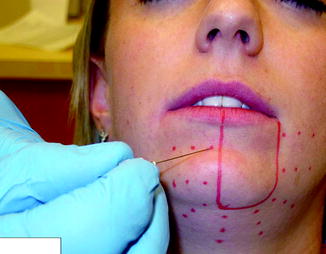

Fig. 10.7
The “marching needle” technique is used to determine the boundaries of the area of altered sensation in a patient with complaints of left lower lip and chin numbness after lower third molar removal. A 27-gauge needle is used beginning in an area of normal sensation, and multiple contacts are made (red dots) every few millimeters until the patient reports a change in the sensation (e.g., “sharp” changes to “dull”). After these determinations have been made from the left, right, and inferior to superior, the border of the affected area can be delineated (solid red line)
When performing NST, it is important to understand the concept of threshold of response [60]. When a stimulus (such as a needle) is applied to the skin or mucosal surface, it is done initially with little minimal pressure and with no indentation of the surface tissue. If the patient responds to the stimulus (raised ipsilateral hand), it is noted that the response was at the normal threshold. If the patient does not feel the stimulus, then the stimulus is applied again with just enough additional pressure to produce indentation, but not piercing, of the skin or mucosa. If the patient now responds to the stimulus, this response is noted to be at an increased threshold. This is an abnormal response indicating that the nerve has sustained injury but still has the ability to transmit electrical impulses from the periphery to the CNS. However, that ability is compromised in terms of the numbers of axons able to transmit and/or their speed of transmission (Fig. 10.8). If the patient still fails to respond at the increased threshold, it is noted that there is no response (NR), and no further additional pressure is applied to the stimulus. To further increase the pressure applied to the stimulus (i.e., needle) at this juncture will induce penetration of the skin or mucosa with bleeding and will add no helpful information. This concept produces a simple, but accurate and reproducible, measurement of responses to static light touch and pain (level B and level C testing, see below). Other methods will be described as well.
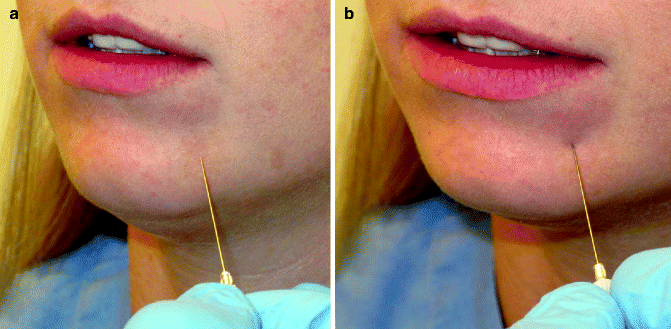

Fig. 10.8
Grading the threshold of applied pressure necessary to elicit a response. (a) A 27-gauge needle is placed in light contact with the skin of the left chin without indenting the skin surface. If the patient responds to the stimulus, this is a response at the normal threshold. (b) If the patient does not respond at this threshold, additional pressure is applied to the needle sufficient to indent the skin without piercing it. If the patient now responds, this is a response at an increased threshold
The NST of a patient with decreased altered sensation differs from that of the patient who complains of unpleasant altered sensation. The goals for diagnosis and treatment are not the same for these two categories of sensory nerve injury patients. In the former (decreased altered sensation), the clinical objective is to improve or restore lost sensory function, whereas, in the latter, reduction or relief of pain is the primary reason for treatment. Therefore, the evaluation of each of these two types of sensory nerve injury patients is discussed separately.
10.5.1 Decreased Altered Sensation
Three levels of NST are available for the patient with decreased or altered sensation without pain. The objective of testing for this type of nerve injury patient is to assess the level of impairment of sensory function as normal, mild, moderate, or severe hypoesthesia or complete loss of sensation (i.e., anesthesia). The tests are done in the order discussed below, and one level of testing may or may not lead to another, depending on the patient’s responses.
Level A testing evaluates spatiotemporal perception which is an indirect assessment of the function of the larger diameter, myelinated, slowly and rapidly adapting A-alpha sensory nerve fibers (5–12 um diameter). Directional discrimination (moving brush stroke identification, MBSI), static two-point discrimination (2PD), and stimulus localization (SL, to assess for the presence or absence of synesthesia
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


