Note: For further discussion, please see Volume II, Chapter 52 Chronic Facial Pain: Evaluation, Differential Diagnosis and Management Strategies by James Swift.
The oral and maxillofacial surgeon is often challenged by the patient with chronic head and neck pain. By its nature, surgery is oriented toward short-term problem solving for patients whose needs are primarily surgical. Since the 1970s, however, it has become clear that there are major differences in the pathophysiology and psychology of acute versus chronic pain and that the surgeon can have major impact on the cause, prevention, or treatment of either form of pain. To be successful in managing chronic pain, the clinician must detect and measure sources of ongoing trauma or noxious disease, assess the status of both the peripheral and the central nervous systems (CNSs) of afflicted patients, and be prepared to use the full spectrum of surgical, pharmacologic, physical, and behavioral therapies that are currently available. It is also apparent that the broad dimensions of the chronic pain condition often require the perspectives of different medical and surgical disciplines. Some patients may be effectively managed by a single clinician; some require simple communication between two or three clinical colleagues or a small collaborative ad hoc group of clinicians; still others may benefit from a formal multidisciplinary pain clinic environment. No single venue is either practical or appropriate for all patients with chronic pain.
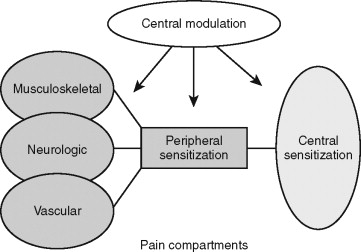
This chapter primarily addresses chronic pain, meaning sustained or recurrent pain states. It does not attempt an encyclopedic presentation of all chronic head and neck pain conditions. Such presentations are available in the two classic general texts in this field by Bonica and by Wall and Melzack and the classic work on orofacial pain by Bell, the proceedings of the International Association for the Study of Pain (IASP) and the International Headache Society (IHS), and in key scientific journals, notably the journal Pain , The Clinical Journal of Pain , and Journal of Orofacial Pain . This chapter targets chronic pain syndromes that have origin in three peripheral anatomic “compartments”: musculoskeletal, trigeminovascular, and neurologic ( Figure 9-1 ). It will show how all chronic pain syndromes originating in these compartments have a “final common pathway” of a sensitized nervous system, how the clinician can recognize this sensitization, and how central nervous dysfunction has implications for patients with all forms of sustained pain. This chapter is heavily referenced to enable readers to pursue topics in greater depth as needed, particularly with regard to the basic mechanisms and modern therapies for pain syndromes.
▪
INCIDENCE AND DEMOGRAPHICS
Chronic pain is a major health care problem, causing untold suffering to the individual and major disruption of work productivity. It is estimated that 30% of the adult population in the United States suffers from some form of chronic pain, and estimates of the cost to society from work loss alone are $80 million per year. If headache is added to this formula, the overall incidence of chronic pain in the American population is more than 60%. Chronic orofacial and TM pain occurs yearly in approximately 22% of the American population. Collectively, patients who come to clinicians for treatment of head and neck pain are more likely to be female, with the female-to-male ratio being 3 : 1. They also tend to be of low socioeconomic status and younger, with peak pain prevalence occurring in mid-30s to -40s. A further breakdown for particular syndromes reveals a female-to-male ratio of 8 : 1 for TM myalgias, 3 : 1 for arthralgias, 2 : 1 for migraine or tension headaches, and 2 : 1 for neuropathic pain.
▪
PAIN DEFINED
Pain is defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. Each individual learns the application of the word through experiences related to injury early in life.” Pain can further be divided into acute, chronic, nociceptive, and neuropathic types. Acute pain is pain of short duration, from noxious disease or recent injury. Acuity does not mean severity. Indeed some chronic pain conditions, such as trigeminal neuralgia (TN), are clearly more intense than most recent-onset surgical pains. Chronic pain is pain of longer duration, usually 3 months or longer, although the neurophysiologic and behavioral changes that accompany chronic pain are often well established within 2 to 4 weeks after pain onset. Nociceptive pain refers to pain of nonneural origin, in which normal peripheral nerve terminals are activated by inflammation or trauma that acts in tissues, such as skin, teeth, muscles, glands, and blood vessels. The modern concept is that no matter where or what the initiating noxious sources, the final conduit for pain is an activation of a specific class of neural receptors known as nociceptors . Therefore, as stated by Devor, “bones don’t hurt, muscles don’t hurt, nerves hurt.” If the underlying causes of nonneural painful disease or trauma are not controlled, a transition from acute to chronic pain may occur. This appears to be due to a series of gene-induced plasticity of transmitting peripheral nerves and ascending central relay neurons, whereby nociceptive pain becomes “centralized” as neuropathic pain through a process known as sensitization . Neuropathic pain, therefore, is a chronic state in which the nervous system has been sensitized by repetitive direct or indirect injury or uncontrolled nociceptive disease. Patients with chronic pain, whether the original nociceptive event was mucosal, cutaneous, muscular, dental, vascular, or neurologic, all appear to share certain common features of neuropathic pain, specifically: (1) lowered pain thresholds, (2) spontaneous and elicited (triggered) pain activity, (3) central behavioral changes, and (4) refractoriness to therapies that would ordinarily relieve acute nociceptive pain ( Table 9-1 ).
|
▪
PAIN DEFINED
Pain is defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. Each individual learns the application of the word through experiences related to injury early in life.” Pain can further be divided into acute, chronic, nociceptive, and neuropathic types. Acute pain is pain of short duration, from noxious disease or recent injury. Acuity does not mean severity. Indeed some chronic pain conditions, such as trigeminal neuralgia (TN), are clearly more intense than most recent-onset surgical pains. Chronic pain is pain of longer duration, usually 3 months or longer, although the neurophysiologic and behavioral changes that accompany chronic pain are often well established within 2 to 4 weeks after pain onset. Nociceptive pain refers to pain of nonneural origin, in which normal peripheral nerve terminals are activated by inflammation or trauma that acts in tissues, such as skin, teeth, muscles, glands, and blood vessels. The modern concept is that no matter where or what the initiating noxious sources, the final conduit for pain is an activation of a specific class of neural receptors known as nociceptors . Therefore, as stated by Devor, “bones don’t hurt, muscles don’t hurt, nerves hurt.” If the underlying causes of nonneural painful disease or trauma are not controlled, a transition from acute to chronic pain may occur. This appears to be due to a series of gene-induced plasticity of transmitting peripheral nerves and ascending central relay neurons, whereby nociceptive pain becomes “centralized” as neuropathic pain through a process known as sensitization . Neuropathic pain, therefore, is a chronic state in which the nervous system has been sensitized by repetitive direct or indirect injury or uncontrolled nociceptive disease. Patients with chronic pain, whether the original nociceptive event was mucosal, cutaneous, muscular, dental, vascular, or neurologic, all appear to share certain common features of neuropathic pain, specifically: (1) lowered pain thresholds, (2) spontaneous and elicited (triggered) pain activity, (3) central behavioral changes, and (4) refractoriness to therapies that would ordinarily relieve acute nociceptive pain ( Table 9-1 ).
|
▪
PAIN PATHOPHYSIOLOGY
A molecular basis for understanding the common features of chronic pain sensitization in all anatomic compartments has been emerging since the 1970s. Sensitization appears to occur at both the peripheral and CNS ( Figure 9-2 ). In the peripheral sensitization of trigeminal nerves, small A-delta-fiber and C-fiber nociceptors become chronically hyperreactive to all stimuli because of at least three interacting processes. A first mechanism of peripheral sensitization is the sustained release of inflammatory chemical mediators, such as 5-hydroxytryptamine (5-HT), bradykinin, and prostaglandins at the nociceptor surface receptor and by the sensitized trigeminal ganglion. As a result, nociceptor thresholds appear to decrease such that stimuli that would otherwise not be painful, such as touch or pressure, are transmitted to the CNS through the sensitized nociceptors and ultimately perceived as pain.
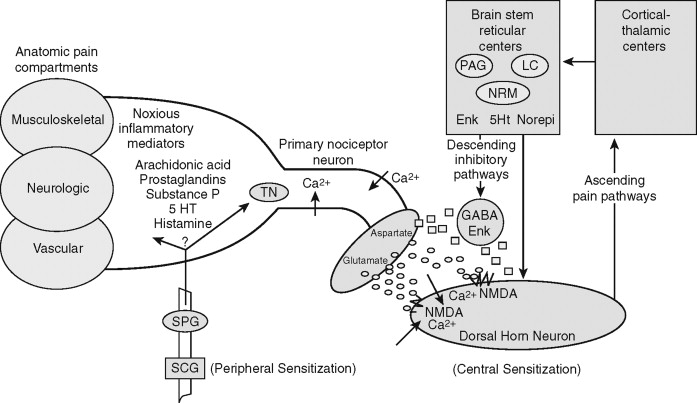
A second mechanism of peripheral sensitization appears to be the induction of pathologic ectopic electrophysiologic activity at specific sites on injured nerve trunks (neuromas) or the trigeminal ganglion itself. A third mechanism of nerve sensitization has also been linked to pathologic changes in autonomic nerves, which become neurochemically linked to injured peripheral somatosensory nerves through sprouting or up-regulation of autonomic receptors. Collectively, these three mechanisms of peripheral sensitization appear to alter the neurochemical and electrophysiologic status of the primary afferent fibers that synapse on second-order neurons in the dorsal horn regions of the brainstem-trigeminal complex.
Central sensitization, a feature common to all forms of chronic pain, appears to be an increase in the tonic excitability of wide dynamic range (WDR) neurons in the dorsal horn of the spinal nucleus of cranial nerve V (see Figure 9-2 ). The WDR neurons appear to become especially permeable to CA + influx as a response to the action of certain key excitatory amino acids, particularly glutamate, arriving in excess at the postsynaptic WDR neurons from the sensitized peripheral nerve. The actions of glutamate appear to be mediated by an important receptor, the N -methyl-D-aspartate (NMDA) receptor. NMDA-receptor antagonistic drugs have been shown experimentally to block neuropathologic forms of pain that are refractory to usual therapeutic levels of opioids, and they hold promise for future treatments of intractable chronic pain.
Similar sensitization mechanisms have now been demonstrated in key central relay pathways and nuclear centers. Plasticity and sensitization have been exposed through PET scan imaging in the posterolateral thalamus and the somatosensory cortical centers that mediate the sensory-discriminative qualities of pain. Neuroplastic sensitization has also been demonstrated in the medial thalamus, amygdala, and anterior cingulate cortex, mediating centers for the affectiveemotional (suffering) aspects of chronic pain. Most recent brain imaging research has shown that the insular cortex may be a key integrating center for pain perceptions and responses. The phylogenetically ancient insula is somatotopically organized and positioned for mediating sensory-discrimination and affective-emotional pain components and activating visceral-motor pain reactions ( Figure 9-3 ).
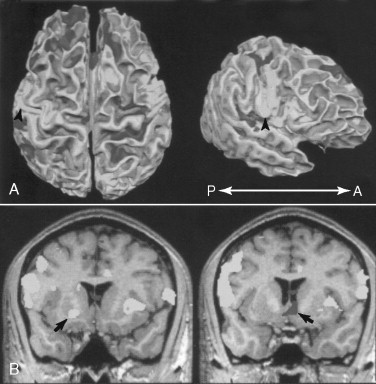
Central sensitization may also result paradoxically from loss of sensory input to the brainstem as occurs with peripheral nerve damage in addition to resulting from CNS lesions, such as stroke or demyelinating disease. This condition, known as deafferentation pain , results in pain and phantom phenomena experienced by patents in peripheral nerve distributions that have been “deafferentated” and that will test deficient on neurosensory examination.
A final basis for chronic central sensitization appears to be failures in the brain’s descending pain inhibition systems that originate in forebrain-hypothalamic centers, descend to spinal cord brainstem levels, and modulate pain through neurochemical mediators, such as 5-HT, norepinephrine, GABA-nergic, and enkephalinergic interneurons. Loss of pain inhibition may account for the protracted pain often experienced by patients who have sustained head and neck injuries. They are also implicated in the generalized neurobehavioral disability often seen in patients with chronic pain.
In addition to the generalized effects of nervous sensitization, certain gross anatomic features of the maxillofacial region may contribute to the uniqueness of this region’s pain syndromes. The muscles of mastication and the neck are interdependent and, when involved in pain syndromes, often present combined symptoms. Arteries and venous plexuses are encompassed at many levels by muscles, such as those of the temporal fossa and pterygoid regions, making the vessels susceptible to extremes of compression from muscle overuse syndromes. Motor and sensory trigeminal nerves are not anatomically mixed, as in spinal systems, making it more likely that head and neck disease will cause painful conditions without accompanying motor signs. The sensory trigeminal nerves often course through nondistensible bony canals and foramina, predisposing them to compressive or inflammatory neuropathies. Finally, the fiber spectrum of the trigeminal sensory nerves as compared with spinal nerves is heavily skewed toward larger, rapidly conducting myelinated fibers, a possible explanation for the occurrence of shocking, more rapid paroxysmal pains seen in the maxillofacial region in contrast to the duller, deeper pains experienced in other body regions.
▪
PAIN DIAGNOSIS
The IASP classification of head and neck pain disorders includes 55 different pain conditions for the head and neck. In recent years, pain taxonomists have suggested that pain syndromes should be grouped according to similar mechanisms of action. This is useful for effective treatment planning and for developing new therapies. However, the clinician is more likely to be successful in treating patients if pain conditions with common mechanisms are also identified within anatomic compartments because this parallels the manner in which patients are logically examined and commonly treated. The anatomic compartments in which chronic pain syndromes most commonly manifest are overlapping combinations of the musculoskeletal-articular, trigeminovascular, and neurologic, with the central nervous compartment acting as a constant in all cases (see Figure 9-1 ). The primary chronic pain syndromes, listed in the contexts of their anatomic compartments, are displayed in Table 9-2 .
| Anatomic Compartment | Pain Syndrome |
|---|---|
| Musculoskeletal | FM |
| Myofascial pain disorders | |
| TM arthralgias | |
| Trigeminovascular | Migraine |
| Tension-type headache | |
| Cluster and autonomic cephalalgias | |
| Orofacial migraine variants | |
| Neurologic | Classic TN |
| Postherpetic TN | |
| Postconcussion syndrome | |
| Posttraumatic TN | |
| CRPS | |
| Idiopathic Orofacial Pain Syndromes |
In any listing of pain entities of the maxillofacial region, it must be recognized that for many pain syndromes the etiologic mechanisms and the balance of peripheral and central factors is not completely known; hence a number of “idiopathic” disorders are grouped in the text and discussed with vascular and neurologic disorders. Using this anatomic-compartment and mechanism-based orientation, the clinician’s diagnostic tasks are to: (1) characterize and quantify pain, (2) determine the presence or absence of ongoing noxious pathoses or chronic painful dysfunction in each of the anatomic compartments, and (3) recognize the signs of peripheral and central sensitization.
▪
CHARACTERIZING AND MEASURING PAIN
A first goal of chronic pain assessment is to determine the nature and severity of the presenting pain and its related function impairment. These determinations serve as the basis for judging the outcomes of treatments used. Although no practical objective technique exists at this time for measuring pain, indirect methods using verbal descriptors and visual analog scaling have proved useful for many types of chronic pain conditions across many cultures. The McGill pain inventory is a well-tested instrument that combines a visual analog with weighted pain descriptors. The pain descriptors of the McGill pain inventory often assist in diagnosis by exposing qualitative features that are unique to particular syndromes. For example, patient selection of descriptors, such as “shocking, flashing, tingling, intermittent” point toward neuralgia; “throbbing, pulsing, nauseating, pressure” more likely point toward vascular pain sources; and “pinching, pulling, tight, tired” direct the clinician toward musculoskeletal compartment syndromes.
Function impairment associated with the pain syndrome can also be ranked on visual analog scales for global life activities, such as work, career, family, marriage, and leisure. It can also measure the perceived impact of pain on dysfunction for specific personal functions, such as sleep, eating, appetite, sex, and maintenance of personal and oral hygiene.
▪
CLINICAL AND LABORATORY EXAMINATION
The clinical examination begins with classic inspection, palpation, and percussion of the patient’s head and neck. The clinician searches for signs of ongoing noxious pathoses that might indicate active inflammation, infection, tumor compression, repetitive trauma, neoplasm, or glandular obstruction. The dentition, oral soft tissues, paranasal sinuses, and salivary and lymph glands should be examined with classic techniques to rule out active noxious pathoses that may be sources of acute or chronic episodic pain.
When acute sources of noxious pathoses have been ruled out and have been cataloged, clinical indicators associated with chronic pain are researched. Atrophy, asymmetry, cutaneous discoloration, herpetic lesions, mucositis, and hyperkeratosis are commonly associated with chronic syndromes. The musculoskeletal compartment is specifically addressed by palpating all muscle groups, especially the trapezius, sternocleidomastoid, occipital, masseteric, temporal, and medial and lateral pterygoid groups. They are tested for painful tender points, trigger points with classic muscle fasciculations, and restricted or deviant range of motion. The range of motion for the neck is observed for flexion, extension, and rotation, and the upper spine is palpated for signs of elicited pain or crepitus. The TM capsule is percussed, and pain responses are noted to both transmeatal and preauricular percussion. Crepitus, popping, and clicking joint effects are recorded for both manual and auscultatory signs.
The vascular compartment is examined with classic inspection, palpation, and percussion of vascular and related tissues, beginning with the carotid sheath in the neck and extending to the pericranial vessels. Pain responses to palpation and pain radiating distally into the jaws and face suggest association with adenitis in the salivary or lymphatic glands through which the vessels course. Auscultation for bruits may further define a carotid atherosclerotic contribution to chronic pain and may signify generalized connective tissue disorder. Doppler analyses may be useful in further defining the extent of vascular disease and its possible implication in the pain syndrome. Inspection of mucocutaneous tissues for erythema and asymmetric edema may signify either vasculopathy or autonomic derangement, or both. It is also important to establish, through vascular analyses and neurosensory testing, the basis for a patient complaint of regional “numbness.” This aspect of the examination should differentiate between true trigeminal nerve branch stimulus-response deficits as compared with normal neurosensory reflexes within an area sensed as “numb” because of direct neural effects of vasoconstriction or reduced blood flow. The patient with a vascular basis for subjective numbness tests normally on trigeminal quantitative sensory testing. Correlation should also be made between headache patterns and ophthalmologic signs, such as increased intraocular pressures and, more globally, hypertension.
The neurologic compartment is assessed on a central to peripheral nervous continuum. The examiner begins with assessment of global functions: level of consciousness, cognitive functions, speech, status of deep tendon reflexes, and signs of quadrant paresis or loss of sensation that may point to central lesions. Cranial motor nerve functions are checked, especially noting deficits in pupillary accommodation reflexes and extraocular muscle coordination. The purpose here is to detect gross signs of generalized neurologic dysfunction as a possible basis of or contributor to chronic pain, such as space-occupying intracranial lesions, demyelinating-proliferative disease, or disseminated metabolic or neuroendocrine disease (such as pituitary, thyroid, or pancreatic disease).
The examination then proceeds to a study of craniofacial stimulus responses known as quantitative sensory testing (QST), noting signs of deficits (anesthesia or hypoesthesia) or excessive neurologic “trigger responses” (hyperesthesia). This examination is done by presenting a cascade of stimuli bilaterally, beginning with level A testing, a measure of patient ability to detect fine-touch stimuli, such as cotton wisp, two-point touch, or brush direction accuracy. The neurosensory examination then proceeds to a measure of patient capacity with crude touch, such as pin-touch detection or the Semmes-Weinstein (von Frey) thresholds (level B testing) and finally to responses and detection levels to noxious stimuli, such as heat, pinch, or deep pin (level C testing). Patients in whom hypoesthetic neuropathies have developed from peripheral nerve injuries or a metabolic disorder demonstrate elevation of all thresholds throughout the cascade but especially for fine-touch (large fiber) discrimination. Despite having elevated thresholds, even for stimuli that are noxious, many patients with neuropathic pain syndromes complain of pain experienced “deeply” within the hypoesthetic tissue regions. Patients who demonstrate signs of neural sensitization display lowered thresholds to the stimulus cascade and indicate that their pain is “on the surface” when compared with contralateral nonpainful distributions. Some patients may describe instantaneous mucocutaneous pain in response to light (levels A and B) stimuli that are not painful in their contralateral nonpainful tissues. This phenomenon of touch-evoked pain is known as allodynia . Other sensitized patients report hyperalgesia , or noxious-stimulus pain, experienced at levels that are elevated when compared with noxious stimulation of contralateral tissues. Hyperpathia is another response signifying neural sensitization, in this case from repetitive mechanical stimulation. Finally, QST may demonstrate spreading of pain sensitivity from the originally lesioned nerve distributions, known as secondary or surround hyperalgesia . At the completion of the neurologic compartment assessment, the clinician should have localized sources of nociceptive and neuropathic pain and determined whether there are signs of peripheral or central sensitization.
▪
PHARMACOLOGIC SCREENING
Pharmacologic and block testing have the dual purposes of helping define the chronic pain mechanism and predicting the success of specific pain therapies.
LIDOCAINE TESTING
Topical lidocaine . Can be used in diagnoses either through topical gels applied to tender points and pain triggers or through overnight testing with transcutaneous 5% patches (Lidoderm). These agents may also assist in palliative pain control.
Paraneural lidocaine . Block injections are done, beginning in the most distal nerve branches with small concentrations and noting reports of pain relief from the patient. Secondary and tertiary blocks are then repeated at higher levels of the trigeminal nerve branch being tested, noting the patient’s report of perceived pain changes after each block. This technique defines the neuropathic contribution to the patient’s pain mechanism and may assist in the treatment planning for surgical decompression in cases of suspected traumatic neuroma or compression neuropathy or control of classic TN.
Intravenous lidocaine . It is possible to learn about a given patient’s locus of pain generation by noting pain responses to intravenous lidocaine. In this test, placebo saline is given first to check for placebo pain response, followed by intravenous lidocaine infused at 1 mg/kg body weight over a 2-minute time frame. At 30-second intervals, patients are asked to rank the severity of their pain, noting changes in its intensity or its quality. A drop in pain severity of greater than 30% is considered a positive response, signifying a neuropathic basis of pain as compared with other sources, such as peripheral musculoskeletal or vascular inflammation. It may also be predictive of pain responsiveness to centrally acting drugs, such as anticonvulsant agents. Although the intravenous lidocaine test has been proposed as a primary screening technique for central neuropathic pain, it should be noted that peripheral neuropathic pain generators, such as active neuromas, are also responsive to higher levels of systemic lidocaine.
Myofascial lidocaine blocks . Diagnostic blocks of muscle myofascial tissues with plain lidocaine may define more precisely the loci of myofascial tender points. Key targets for diagnostic blocks are the occipital and masticatory muscle groups, which may differentiate pain that is exclusively myofascial in origin as compared with spinal or temporomandibular-articular sources.
Autonomic blocks . Both sympathetic and parasympathetic components of the autonomic nervous system can be tested for possible involvement in chronic pain syndromes. Stellate ganglion blocks are done at the C7 level with 10 mL lidocaine and induce a hemifacial block of postganglionic sympathetic fibers to the ipsilateral maxillofacial structures. Signs of effective block include skin warming, pupillary dilatation, and eyelid ptosis. If pain is sympathetically mediated, the patient’s chronic pain will be transiently relieved, although there will be little effect on trigeminal nerve or myofascial sources of pain.
The sphenopalatine ganglion may be blocked either directly at the posterior junction of the inferior and middle turbinates through transnasal 4% lidocaine insufflation or through transpalatal injection into the descending palatine canal. Pain remission may point toward a vascular pain syndrome because of the possible parasympathetic role of the sphenopalatine ganglion in craniofacial migraine and migraine-equivalent syndromes. However, because of the proximity of the maxillary division of trigeminal nerves in the sphenopalatine region, it may not be possible to differentiate between pain sources from TN and migraine with the sphenopalatine ganglion block.
▪
SYSTEMIC DISEASE, SLEEP, AND PSYCHIATRIC DYSFUNCTION
Chronic pain does not exist in a demographic vacuum. The patient’s general health and psychosocial factors act to some degree in all cases and can be primary determinants of pain severity reporting, pain tolerance, and responses to therapy. Chronic pain is known to be influenced by patient age, gender, socioeconomic status, educational level, family and marital status, religious and cultural background, previous life experiences with chronic disease, particularly painful disease, the presence or absence of litigation, levels of daily exercise, and avocation satisfaction.
Systemic disease . Assessing past medical history is important because chronic oral and maxillofacial pain is known to cluster with other generalized pain conditions. There are common associations between arthritis, fibromyalgia (FM), irritable bowel syndrome, migraine, hypertension, chronic paranasal sinus pain, and cervical spinal and low back pain. Although no common mechanisms have been proven for these painful conditions, similar psychiatric patient profiles and known reflex connections between autonomic and trigeminal systems have been implicated.
Sleep dysfunction . A detailed history of the patient’s sleep history is important, especially with regard to its relationship to pain. The patient should be asked whether he or she is awakened by pain or simply notices pain on awakening. Musculoskeletal pain exacerbations tend to awaken individuals, as does neuropathic pain where a cutaneous trigger is present. Intracranial-based pain, such as TN, however, is more commonly “dormant” during sleep and does not awaken the patient. Patients lacking in deeper sleep levels III and IV and rapid eye movement (REM) sleep are prone to myofascial pain and FM patterns. Others with restless leg syndrome may have more intense oral dyskinesias associated with clenching or bruxism. Patients with obstructive sleep apnea syndrome may have many features of FM and oral dyskinesias, and there is known correlation between nocturnal bruxism dyskinesias and smoking.
PSYCHIATRIC DISORDERS
A detailed history for previous and current psychiatric conditions should be discussed openly with patients and family. Three psychiatric diagnoses are of particular relevance to chronic pain: drug addition, depression, and somatoform disorders.
Substance abuse . Abuse and addiction to tobacco, alcohol, opiates, psychosedatives, or other centrally acting drugs is more common among patients with chronic pain than in the pain-free population. The paradigm for use of long-acting opiates for management of all forms of chronic pain has changed in the last 2 decades and now recognizes the appropriateness and safety of this practice. Current and past use of any centrally acting drugs must be discussed openly with patients, and a substance use contract should be agreed upon between the patient and treating physician before treatment begins.
Depression is by far the most common psychiatric illness found in people with chronic pain, although it is believed that depression rarely manifests itself as pain. Rather, depression appears to result from the demoralizing and debilitating aspects of chronic pain and profoundly affects vital functions, such as sleep efficiency, which in turn has major impact on pain tolerance and musculoskeletal and vascular pain cycles. Patients with a history of depression should be questioned for details of whether depression has a familial basis; whether it has been associated with substance abuse; whether it has been endogenous or situation dependent; has had bipolar features, including self-destructiveness; and whether hospitalizations have occurred.
Somatization is the tendency to experience and communicate somatic distress and symptoms that cannot be explained on a physical basis. Patients with chronic pain are known to catastrophize symptoms, apparently in some cases to convince others of the truthfulness or severity of their symptoms, and consequently they seem hypochondriacal.
The clinician may be assisted in screening for psychiatric relations to pain by the use of certain instruments. Of proven value are Beck’s Depression Inventory, Spielberger State-Trait Anxiety Inventory, SCL90, and Pittsburgh Quality of Life Inventory. More extensive and long-established psychological screening instruments, such as the Minnesota Multiphasic Personality Inventory (MMPI), although helpful in defining psychological disease trends, require professional psychological assistance for full interpretation.
Social dysfunction . Chronic pain commonly disrupts patients’ relationships with spouse and family, job performance, and community engagement. Failure in these spheres further impairs the patient’s tolerance for ongoing pain, may lead to further destructive behavior, and jeopardize possible therapies. It is critical that the clinician gain knowledge of these dynamic factors before beginning treatment.
▪
THE MUSCULOSKELETAL COMPARTMENT
Pain derived from the musculoskeletal compartment of the head and neck are the chronic syndromes most frequently seen in clinical practice comprising 40% of all chronic cases seen in pain clinics. Muscle trauma and overuse, joint inflammation, and mechanical disk derangements are clear peripheral sources of pain and often account for the isolated and episodic pain conditions seen in this compartment. The more refractory and chronic forms of musculoskeletal pain syndromes, however, are more likely associated with central sensitization patterns similar to those seen in neuropathic and vascular syndromes. There is much overlap, continuity, and common pathophysiology between musculoskeletal pain that is generalized and syndromes that are confined to the back, cervical, masticatory, and TM regions. There is also overlap with vascular and neurologic compartment syndromes, particularly migraine variants and “atypical” or idiopathic facial pain syndromes. Three primary syndrome types dominate the musculoskeletal compartment: (1) FM, (2) myofascial pain syndromes of the head and neck, and (3) the TM arthralgias.
▪
FIBROMYALGIA
Fibromyalgia (FM), also known as primary fibromyalgia syndrome, is a common condition of muscle aching, stiffness, generalized fatigue, and nonrestorative sleep pattern with multiple myofascial “tender points” throughout the trunk and appendages. Pain is described as “aching, radiating, gnawing, shooting, and burning,” is worse in the mornings on awakening and later in the evening, and is exacerbated by cold temperatures and physical activity. Patients complain of both spontaneous deep muscle aching and localized tender-point pain responses to 4 kg of digital pressure. Studies have demonstrated a generalized reduction in muscle pain thresholds for pressure pain. Sleep onset and retention are poor and are associated with daytime sleepiness and work disability.
FM is believed to occur in approximately 2% to 4% of the population, with 80% to 90% being female with a mean age of 52. There is also considerable overlap between FM and TM and cervical myofascial pain. Of FM patients, 75% also have TM-myofascial pain, and approximately 30% of patients diagnosed with TM pain are believed to have FM. There appears to be a hereditary predilection. FM is best distinguished from specific myofascial pain syndromes, such as cervicogenic and TM syndromes, by the presence of multiple thoracic and appendage tender points.
PATHOPHYSIOLOGY
A central nervous pathophysiology accounts for the main elements of FM. Psychological theories have suggested that patients amplify unpleasant experiences and are hypervigilant to painful events. Studies of serotonin metabolism have also shown that patients with FM may have deficiencies in CNS pain modulation owing to serotonin pathway dysfunction. Others have postulated that chronic myofascial pain is due to dorsal horn sensitization induced by increased input from myofascial nociceptors.
DIAGNOSIS
FM diagnosis is dependant on the demonstration of at least 11 trunk and appendage tender points. Rheumatoid factor analyses are normal. Patients have significantly lowered pain thresholds for heat and pressure and an incapacity to inhibit secondary pain stimuli at normal levels. The overall clinical pattern is suggestive of pituitary derangement and a deficient generalized stress response. FM also has many features in common with hypothyroidism, but patients test euthyroid, and adrenocortical functions are depressed with reduced cortisol levels. Unfortunately, no definitive laboratory or imaging studies aid in the differential diagnosis of FM.
TREATMENT
There are no universally definitive treatments for FM, and few patients become permanently pain free. Treatment programs rely on pain-management counseling for patient self-management and lifestyle modification. Medical treatments depend on simple nonsteroidal analgesics and the titrated use of low-dosage tricyclic antidepressant agents to increase pain tolerance thresholds and normalize sleep. Tender-point injections with local anesthetics and corticosteroids and spray and stretch physical therapy may bring about sustained remissions. Intravenous lidocaine (administered weekly at 4 mg/kg infusions) has proven effective in relieving pain symptoms of chronic FM pain for approximately 1 month and can then be supplemented by the use of titrated oral mexiletine. Although regular aerobic physical exercise is generally recommended for chronic pain management, isometric exercise has recently been shown to have a hyperalgesic (pain exacerbation) effect on patients with FM.
▪
MYOFASCIAL PAIN
The extremely common myofascial pain syndromes of the head and neck appear to be interdependent, have overlapping symptoms and courses, and probably share common pathophysiologies. The three main recognized head and neck myofascial pain syndromes are the temporomandibular myofascial syndrome, the cervicogenic (occipital) headache, and the overlapping neurovascular tension-type headache.
TEMPOROMANDIBULAR DISORDERS
Temporomandibular myofascial pain and dysfunction (TMD) is a chronic episodic condition in which patients experience pain in the muscles of mastication and surrounding structures, often in association with pain in the occipital, cervical, and upper back muscles. Pain is usually described as preauricular and temporal and often radiates anteriorly to precipitate retroorbital headaches with photophobia and nausea. TMD overlaps with primary occipital myalgias; cervicogenic, mixed tension-type headaches; and so-called “atypical” midfacial pain patterns. The TM and cervical regional syndromes are distinguished from primary FM by pain being confined to the head and neck region, by distinct patterns of referred pain, and by specific “trigger points,” where muscles have taut, palpable band regions that twitch when manually percussed. There is also an association with TM joint sounds of clicking, popping, and crepitus, which are highly variable between patients and also vary over time for individual patients. Intraarticular symptoms are often associated with myofascial pain symptoms, and therefore, may represent parallel or interrelated clinical problems (see section on temporomandibular joint disorders). Tinnitus and vertigo are common but inconsistent aspects of TMDs.
TMD is common among Americans, with some aspects of the syndrome present in up to 11 million people, or 6% of the population. Onset is most common in the second decade of life, with peak incidence in the third and fourth decades and lowered incidence and severity after age 50. TMD is characterized by episodes of increased pain and dysfunction severity lasting days to weeks followed by gradual remissions. Like all of the musculoskeletal pain syndromes, TMD is strongly female in its frequency, with female-to-male ratios 6 : 1 to 9 : 1.
CERVICOGENIC HEADACHE
Cervicogenic headache appears to be due to myalgia of the occipital muscles and is possibly related to entrapmentcompression of the greater occipital nerve at the base of the superior nuchal line. Many patients with chronic occipital myalgia have sustained head or neck trauma as an apparent precipitating event. Pain is usually unilateral, dull, daily, constant, and radiating from the base of the neck toward the shoulders to temporal-frontal regions and, in some cases, to midfacial maxillary arterial or trigeminal distributions. The latter cases have historically been linked to “atypical” referred muscular-vascular syndromes that have been attributed to psychogenic factors. A more likely neurophysiologic link, however, is through the cervical and trigeminal primary afferents that converge in the subnucleus caudalis of the spinal cord.
PATHOPHYSIOLOGY
Two universal mechanisms appear to account for sustained myofascial pain syndromes: tissue trauma and central nervous sensitization. Trauma to myofascial tissues of the head and neck is a common pathophysiologic denominator for many of the myofascial pain syndromes. Direct macrotrauma to the jaws, particularly the symphysis or lateral mandible, is known to initiate microstructural focal weakness in muscle tendons and may precipitate chronic pain.
Repetitive microtrauma to masticatory structures, from either iatrogenic trauma, battering, or self-induced overuse activities, such as bruxism, are possible sources of CNS sensitization and pain chronicity. Chronic pain and decreased pain thresholds following microtrauma may cause central “windup” sensitization of dorsal horn NMDA receptors resulting from stimulation of muscle nociceptors by repetitive and summating microtraumas.
Cervical musculoskeletal macrotrauma is commonly associated with both cervicogenic headache and midfacial and TM pain. The mechanisms of this association remain unclear. The theory that indirect trauma, such as rapid extension-flexion cervical trauma (whiplash), can induce permanent internal derangement of the TM joints remains controversial. It has been proposed that indirect whipping action of the cervical, facial, and masticatory muscles can induce focal muscle damage and weakening. Alternative mechanisms involve the central brainstem-trigeminal complex, which may become sensitized by noxious input from injured cervical spine and occipital myofascial injury, or more global posttraumatic brain effects.
Psychosomatic factors have also been strongly implicated as precipitating or exacerbating factors, or both, for chronic myofascial pain, mediated through inadequate stress response (coping), psychiatric depression, and especially dysfunctional sleep disorders, acting as perpetuating and sustaining factors for the muscular pain patterns.
DIAGNOSIS
Diagnosis of head and neck myofascial pain syndromes is dependant on the demonstration of specific painful tender points in muscles, either in response to manual percussion and palpation or with an algometer. Restricted or irregular jaw or neck movements are determined to be muscular rather than articular. Imaging of the mandibular condyles may demonstrate remodeling from long-term masticatory hyperfunction, which can be differentiated from true arthrotic inflammatory imaging changes (see the section on temporomandibular disorders). Cervical spine films may demonstrate osteoarthritic changes, which can be differentiated from more recently acquired and purely soft tissue pain sources. Local anesthetic field blocks in muscles, such as the lateral pterygoid or inferior temporalis attachments, may help define the myologic source of pain. In the case of cervical syndrome, occipital anesthetic blocks done between the mastoid process and the external occipital protuberance will define an occipital myalgia source. In those cases where there is an occipital to midfacial referred pain linkage, the occipital block will also relieve midfacial symptoms. Pain relief from tender-point anesthetic blocks may be predictive of therapeutic benefit from serial blocks, physical therapy, or electrotherapy.
The clinician should carry out quantitative sensory testing for all patients who have musculoskeletal compartment pain to identify signs of central sensitization. Diagnostic signs of central sensitization seen in association with muscle tender points and limited range of mandibular motion would include (1) lowered pain thresholds to all forms of stimulation, (2) spread of pain beyond specific muscle sites, (3) mucocutaneous touch-evoked pain responses (allodynia) and hyperalgesia, and (4) failure of peripheral lidocaine nerve blocks to significantly decrease pain. Recognition of the central neuropathic mechanisms of chronic multiple sclerosis (MS) pain will more correctly direct the therapy toward the primary central mechanisms and prevent reliance on ineffective and potentially destructive treatments.
TREATMENT
Myofascial pain management incorporates behavioral, physical, pharmacologic, and surgical therapies for overall pain management. Treatments must be individualized, with avoidance of lock-step, cookbook approaches. Because myofascial pain is often episodic in nature, some interventions must address acute pain exacerbation, whereas more supportive and self-help measures are needed for maintenance and prevention.
Behavioral management of myofascial pain is based on patient awareness of his or her own role in disease exacerbation and control. Patients should be counseled to understand the importance of a healthy lifestyle, sleep hygiene, exercise, nutrition, and smoking and substance abuse avoidance in preventing and managing their pain condition. Responsibility for therapy and recovery and prevention should be shifted away from the therapist and toward the patient for long-term remission.
Specific behavioral techniques that can be applied in both intervention and maintenance programs include relaxation therapies, biofeedback, especially electromyographic (EMG) and temperature biofeedback, hypnotherapy, and cognitive behavior therapy. There is a proven crossover of therapeutic effects of behavioral-relaxation approaches between musculoskeletal pain and insomnia.
Physical therapy programs can also be of value for patients with myofascial pain syndromes. During phases of acute exacerbation of pain, physical tissue stress can be reduced through use of soft diets, occlusal splinting, and icing of the joint and muscular tender points. For more chronic pain control, formal physical therapy can be prescribed, specifically for electrotherapy using iontophoresis or phonophoresis, and home therapy with TENS applied to tender points. In chronic phases and after joint surgery, regular mandibular and cervical mobilization is extremely important, with the primary goal of improving range of motion through practiced stretching and toning of antagonistic muscles as directed by the physical therapist.
Pharmacologic management must also be matched to the stage of the pain cycles seen with myofascial pain syndromes. In acute exacerbation stages of myofascial pain, the primary drugs are antiinflammatory agents, local anesthetics, muscle relaxants, and in selected cases, opiate analgesics. Nonsteroidal antiinflammatory agents are taken on a schedule of 1200 to 1800 mg/day (acetylsalicylic acid or preferably ibuprofen equivalents). Muscle relaxants. such as benzodiazepines, are useful with low daily dosing and moderate bedtime dosing, such as alprazolam (0.25 mg three times daily and 2 mg at bedtime). Cyclobenzaprine is also an effective short-term agent when used at 5 mg three times daily and 10 to 20 mg at bedtime. This agent facilitates the transition to more chronic maintenance with tricyclic antidepressants because they have a similar chemical structure. Narcotic agents can be effective in reversing more severe and debilitating pain episodes and can be safely used in short-term situations in conjunction with muscle relaxants and physical and behavioral therapies.
For patients with more constant musculoskeletal pain, the tricyclic antidepressant agents (titrated from 10 to 75 mg amitriptyline equivalents) are the most proven medical approach. Patients should be counseled about the gradual onset (2 to 3 weeks) of therapeutic effect, the expected side effects of early sedation and xerostomia, and possible weight gain. Tricyclic therapy for myofascial pain can often be discontinued or reduced to low-dose bedtime maintenance after a 3- to 6-month time frame. The serotonin reuptake inhibitor agents, such as fluoxetine and sertraline, are also effective for many patients with myofascial syndrome pain and may be better tolerated for daytime use. These agents, however, do not have the proven myofascial syndrome pain-relief efficacy of the tricyclic agents, and they do not appear to be as uniformly effective in sleep management, an important factor in pain management of myofascial pain syndrome.
Recently it has been shown that anticonvulsant drugs have efficacy in the medical management of chronic sensitized musculoskeletal pain. Gabapentin (Neurontin), used in titrated doses between 600 and 2400 mg/day has been shown to be effective particularly for patients with multifocal pain triggers, cutaneous hyperalgesia, and touch-evoked pain triggers.
The use of narcotics and muscle relaxant agents for chronic myofascial pain (and other forms of chronic “benign” pain) remains controversial. There appear to be many patients whose pain is well controlled and whose functional status is clearly improved by a daily program of long-acting narcotic and sedative agents, without evidence of increasing habituating drug usage. The prudent clinician will establish a contractual agreement with such patients, however, regarding the use of narcotics and muscle-relaxant agents. The “contract” should stipulate a daily dosage schedule set at realistic pain-controlling limits; avoidance of patient duplication of medications from other physicians; careful combinations of other CNS agents, such as alcohol; and maintenance of nonpharmacologic activities known to aid in the patient’s pain management.
Musculoskeletal tender-point block therapies , using local anesthetic in combination with adrenocorticosteroid combinations, such as bupivacaine-dexamethasone, have proven value. Blocks may afford pain reduction and increase range of motion for 1 to 3 months and may be effectively combined with physical therapy programs that emphasize range-of-motion and low-impact muscle-toning activities.
There is little evidence that irreversible surgical or orthodontic treatments have a significant direct effect on chronic centralized myofascial pain. They may have indirect value, however, if traumatic tissue forces are reduced and mandibular range of motion is increased in the process. Therefore, orthognathic surgery that corrects traumatic malocclusion may help in overall myofascial pain management. Surgical arthrocentesis and arthroplasty may also indirectly influence myofascial pain if concomitant joint pain is an aggravating factor in the myofascial pain syndrome. The prudent oral and maxillofacial surgeon or orthodontist, or both, will stress the supportive role of irreversible therapies in the overall management of patients with myofascial pain syndromes.
▪
TEMPOROMANDIBULAR ARTHRITIS
Intraarticular disease may be a source of radiating preauricular pain and mechanical joint derangements. The two most common painful conditions associated with the TM joint are (1) TM rheumatoid arthritis and (2) TM osteoarthritis.
TEMPOROMANDIBULAR RHEUMATOID ARTHRITIS
Rheumatoid arthritis (RA) is a systemic inflammatory disease of probable autoimmune origin involving many joints, including the TM joint. Approximately 50% of patients with RA have pain in the TM region, and approximately 10% have deforming arthroses of the mandibular condyle, often resulting in progressive malocclusions. The incidence of RA in the general population is 2.5%, with a female predilection of approximately 3 : 1. Peak onset is in the fourth to sixth decades of life. A juvenile form of RA is rare, but potentially devastating in its effects on mandibular growth and joint mobility. Acute episodes of painful intraarticular inflammation leave patients with secondary intraarticular soft tissue adhesions, producing pseudoankylosis, especially for translational and wide opening movements. When the acute inflammatory cycle has abated, often through spontaneous remission, patients may still experience pain from extraarticular myalgia because of the increased masticatory efforts required to overcome the effects of the arthrosis. Cervical spine RA is also often seen in conjunction with TM involvement, and this combination may summate in craniofacial pain.
PATHOPHYSIOLOGY
RA produces pain by initiating a classic intraarticular soft tissue inflammatory response, involving the synovial tissues and adjacent capsule, and ultimately producing pain by peripheral sensitization of C nociceptors. The adjacent preauricular tissues may display allodynia and hyperalgesia into adjacent temporal and facial soft tissues. The actual cause of RA is unknown, although genetic predilection and autoimmune factors have been implicated.
DIAGNOSIS
RA must be differentiated from psoriatic arthritis, systemic lupus erythematosus, spondylitis, and gout. There is also considerable overlap with noninflammatory osteoarthrosis with regard to condylar imaging deformities. However, hypergammaglobulinemia with antibodies positive against immunoglobulin G rheumatoid factor is found with RA in more than 85% of cases and effectively differentiates it from aggressive arthroses. Patients typically display soft tissue swelling in the preauricular regions with surrounding hyperalgesia.
TREATMENT
The treatment of rheumatoid arthralgia is directed toward reducing the pain of both acute and chronic joint pain, preventing radiation of pain into regional myofascial tissues, and mobilization of restricted joint movements. With acute pain exacerbation, antiinflammatory drugs are employed on a scheduled basis and may be supplemented by direct iontophoresis-phonophoresis of local anesthetic and corticosteroid ions into the glenoid fossae. Support should also be given for control of myofascial pain to enable patients to sustain functional movements around the afflicted joints. Surgical treatments are directed toward lysis and lavage of dysfunctional joints, less often toward total joint replacements. For young individuals in whom significant dentofacial deformity has resulted and the inflammatory phases appear to be in remission, joint reconstruction with autologous rib or with total joint replacement may be used to restore lost function.
TEMPOROMANDIBULAR OSTEOARTHRITIS
TM osteoarthritis, also known as degenerative joint disease, is a commonly painful disorder of the joints in which there are combinations of synovitis, destruction and abrasion of articular cartilage, and reactive formation of compensatory bone and adhesions. It is seen much more often in females, appears familial, usually begins in the teenage years, peaks during ages 20 to 40, and decreases in incidence and severity after age 50. Patients appear to go through episodes of specific preauricular joint pain, sometimes called “arthritic events” that last days to weeks and then dissipate or “burnout,” although mechanical problems remain. The condition is also characterized by progressive mechanical problems of decreasing range of motion, popping, crepitus, occasional tinnitus, and vertigo.
PATHOPHYSIOLOGY
The degenerative responses of osteoarthrosis are believed to be due to a failed capacity of a given patient’s articular cartilage to respond to its mechanical stresses. Both macrotrauma and microtrauma can be responsible for initiating the disintegration processes. Therefore, the same traumatic factors that may precipitate myofascial pain caused by masticatory hyperfunction, such as nocturnal parasomnias-bruxism, may also exacerbate TM arthrotic pain. Painful arthrotic events have been attributed to the traumatic induction of a low-grade synovitis of capsular and retrodiskal tissues in which there is an up-regulation of substance P and sensitization of substance P and CGRP-containing neurons. There is also evidence that TM arthrotic pain may be further sensitized by trauma-induced autonomic receptor up-regulation. Patients display allodynia to fine-touch stimulation over the preauricular skin and decreased systemic pain tolerance thresholds, suggesting both peripheral and central sensitization mechanisms possibly mediated by increased synovial fluid concentrations of serotonin and keratan.
RELATIONSHIP TO INTERNAL DERANGEMENT
Chronically displaced TM disks produce joint popping, crepitus, and a variety of mechanical joint problems, especially during opening and translational movements. Patients may manifest “closed locking,” with difficulty in carrying out full mandibular rotation or translation, or open locking, in which mandibular closure to full dental centric occlusion is impaired. Although patients with internal derangement display osteoarthrosis more than 50% of the time, there is no convincing evidence that internal disk derangement is the direct cause of arthrosis. Early arthrotic changes appear to precede disk displacement, and arthrosis occurs in patients with normal disks. Most patients with acute or chronic disk derangements do not have pain, and recent surgical studies have confirmed that many patients treated successfully with intraarticular open or closed procedures enjoy pain remission without correction of disk displacement. Therefore, disk displacements are probably a sign of osteoarthrosis rather than a cause.
DIAGNOSIS
Pain is specific to the preauricular region and increases with lateral pressure, movement, and sometimes clenching. Crepitus or popping is usually palpable and auscultatable, although younger patients may exhibit only pain. Transpharyngeal imaging displays a range of arthrosis from early anterior lipping and subchondral hypocalcification through more major condylar deformities, osteophytes, surface erosions, dense sclerotic zones, subchondral cysts, and, in later stages, marked condylar thinning. Magnetic resonance imaging (MRI) analysis is helpful for establishing the severity of hard and soft tissue joint degenerative change, but it is rarely necessary to establish the basic diagnosis. Diagnostic intraarticular local anesthetic blocks may help differentiate pure arthrotic pain from extraarticular myofascial pain syndromes.
TREATMENT
A single best treatment protocol for painful arthrosis with or without internal derangement remains elusive. Indeed, most therapies seem to demonstrate a high rate of success, suggesting the possibility that “natural processes” may also act in producing remission from painful episodes, if not the degenerative arthrotic changes. The specific treatment emphasis should be individualized, tailored to the stage of the condition, age of the patient, and level of pain severity.
Acute exacerbations in patients with arthrosis should be treated with combinations of antiinflammatory physical and medical therapies. Joint rest with soft splinting, soft diet, and icing of erythematous or edematous joints twice daily is useful. A course of nonsteroidal antiinflammatory agents (1200 to 2000 mg daily of ibuprofen equivalents in three divided doses) should be administered by schedule, using 3 weeks on and 1 week off if gastric tolerance is limited. Narcotic analgesics may be required for selective individuals to maintain functional levels. Sleep enhancement with mild psychosedative agents and muscle pain control with benzodiazepines or cyclobenzaprine are indicated, similar to the treatment of acute-phase myofascial pain. If rapid remission from the painful arthrotic phase does not occur, however, more aggressive physical therapy is indicated. Electrotherapy with iontophoresisphonophoresis two to three times weekly applied to joints and adjacent myofascial tissues can be effective.
More chronic forms of arthrotic pain usually require a sustained antiinflammatory level of daily medications and some form of sleep promotion, such as zolpidem (Ambien) 10 mg for sleep onset and a tricyclic antidepressant (such as 20 to 75 mg amitriptyline) for sustained pain relief. Behavioral modification with emphasis on a healthy lifestyle, avoidance of tobacco abuse, and the practice of regular physical exercise may be important in both pain management and resistance of the destructive arthroses over the long course of this condition.
Surgical management of TM joint disorders will be reviewed in detail in another section of this text (see Israel, HA).
▪
THE VASCULAR COMPARTMENT
The primary pain syndromes that occur in the head and neck vascular compartment are (1) migraine, (2) tension-type headache, (3) trigeminal autonomic cephalalgias including cluster headache, and (4) migraine variants. These syndromes appear to have the common pathophysiologic features of an episodic vascular inflammatory noxious event transmitted to and from the CNS by the trigeminal system and regional parasympathetic fibers. They also have considerable diagnostic, pathophysiologic, and therapeutic overlap with neuropathic and musculoskeletal syndromes.
▪
MIGRAINE
Classic migraine headaches are chronic, recurrent painful conditions that affect 6% of adult males and 17% of females. Two general forms are recognized: classic migraine (with aura) and common migraine (without aura), with a variety of intermediate and altered variants. The pathophysiologic relationships between classic and common migraines, cluster headaches, tension-type headaches, and myofascial pain remain unclear, although it appears that there are key common mechanisms and some overlapping treatments.
Classic migraine with aura is characterized by infrequent abrupt-onset throbbing frontotemporal headaches lasting from 4 to 72 hours with secondary spread to the entire hemicranium. Nausea, vomiting, photophobia, and phonophobia often accompany the pain. Classic migraine is especially distinguished by prepain anxiety and mood swings, which are followed by the classic “aura,” characterized by blurred vision, flickering changes in the visual fields, “curtain” visual effects, and flickering “scotomata.” In some cases, facial paresthesias, mild paresis, and aphasia occur.
Migraine often begins in childhood and is well established by the end of puberty, with peak incidence in the 20 to 40 age range. A hereditary basis is suspected, and a predilection for females is well established. Pain attacks may be precipitated by stressful psychic, physical, or traumatic events. A frequent life pattern is one in which the abrupt classic attacks occur one to four times per month, then diminish in severity and frequency to give way to “transformed migraine” in which pain is milder, more frequent, and with or without aura. In other cases, a “paroxysmal hemicranial” pattern may emerge and become chronic.
Migraine without aura, formerly known as common migraine, is grossly and anatomically similar to the classic form, but differs by lacking the distinct aura and overtly autonomic phases. It is seen two or three times as frequently as classic migraine and tends to be frontal and more often bilateral. Pain is aggravated by mild physical exertion, and pain attacks tend to be more lengthy, in many cases up to a week.
PATHOPHYSIOLOGY
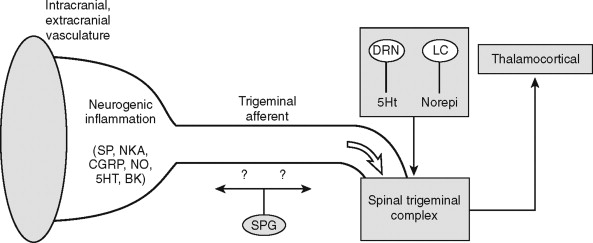
Migraine phenomena appear to be due to the interactive and reciprocal effects of peripheral and central depression and sensitization occurring within the “trigeminovascular system” ( Figure 9-4 ). The peripheral effects occur when cerebral, dural, and meningeal vessels innervated by trigeminal nociceptors become sensitized, possibly from antidromic CNS and autonomic spread to the cranial vessels. The ophthalmic and deep temporal arteries are specifically involved, although these vessels appear to be histologically normal, with no correlation between migraine syndromes and congenital or acquired vasculopathy. In the current model of migraine pathophysiology, termed the “trigeminovascular system,” a cascade of events begins within the CNS, triggered by a variety of events, such as chemical-nutritional, physical trauma, and psychosocial stress (see Figure 9-4 ). The sensitized trigeminal complex, particularly subnucleus caudalis of V, is believed to be stimulated by descending neurochemical influences that arise in forebrain reticular centers, specifically the dorsal raphe nucleus (serotonin) and locus ceruleus (norepinephrine). Through unknown mechanisms, possibly antidromic reflex activity, the trigeminal and parasympathetic ganglia, such as the sphenopalatine ganglion, initiate a neurogenic inflammatory response with the release of substance P, neurokinin A, and CGRP by mast cell degranulation and platelet aggregation. As the final pathway, perivascular trigeminal nociceptor C fibers respond to the neurogenic inflammatory response to mediate the pain response centrally. The final clinical phase of central sensitization is refractory to the triptan class of drugs, but do respond to dihydroergotamines and COX inhibitors. Nitric oxide has been proposed as a key causative molecule in migraine, and interestingly, nitric oxide oxygenase has been found in high concentrations in the sphenopalatine ganglion.
DIAGNOSIS
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


