Introduction
Information about the effect of tooth movement on the myelinated nerve in the periodontal ligament is limited. In this study, we aimed to investigate what responses of the periodontal myelinated nerve can be evoked during experimental tooth movement.
Methods
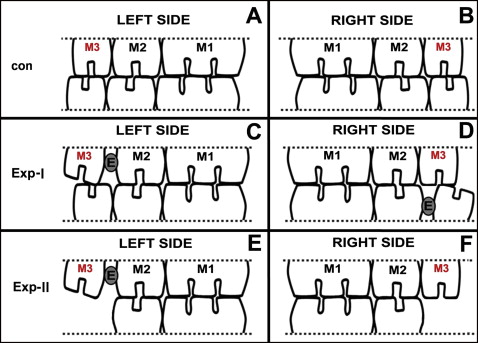
In experimental-I group, the maxillary left and mandibular right third molars were moved distally. In experimental-II group, the maxillary left third molar but not the right one was moved, and the bilateral mandibular third molars were extracted. The ultrastructures of the myelinated nerve in the periodontal ligament of the bilateral maxillary third molars were observed under a transmission electron microscope. The expression of myelin basic protein was evaluated by immunohistochemistry.
Results
Degenerative ultrastructural changes of the myelinated nerve in the periodontal ligament were noticed mainly in the myelin sheath; these were observed earlier and were recoverable in the experimental-I group. In contrast, the ultrastructural changes of the myelinated nerve occurred mainly in the axons, were observed later, and were unrecoverable in the experimental-II group. A concomitant decrease of myelin basic protein expression was observed in both groups.
Conclusions
Both experimental tooth movement and occlusal changes accompanying it caused changes of the myelinated nerve in the periodontal ligament.
The innervation of the periodontal ligament plays an important role in regulating masticatory movement. The myelinated nerve in the periodontal ligament is in charge of transmitting signals to the central nervous system. Tooth movement alters the mechanical microenvironment of the periodontal ligament; this causes a series of biologic reactions : apoptosis of periodontal ligament cells ; remodeling of the ligament, blood vessels, and alveolar bone ; and expression of various regulative factors. In addition, it has been reported that experimental tooth movement can induce ultrastructural or morphologic changes, as well as functional changes of periodontal Ruffini endings, such as decreased mechanical thresholds, discharge frequencies, and conduction velocities. However, little information is available about the effect of tooth movement on the myelinated nerve in the periodontal ligament.
Myelin basic protein is a major component of myelin in the peripheral nervous system and plays a critical role in myelin maintenance and remyelination. It has been shown that shear stress could down-regulate the myelin basic protein level in Schwann cells and induce demyelination and remyelination of injured sciatic nerves in a model of chronic nerve compression injury. It is well known that the mechanical microenvironment in the periodontal ligament can be changed by tooth movement or occlusal changes. However, the influence of the mechanical microenvironment changes induced by experimental tooth movement on the myelinated nerve and myelin basic protein expression in the periodontal ligament has never been investigated before.
Thus far, several animal models of tooth movement have been proposed to study the periodontal changes after tooth movement. Previously, orthodontic elastic bands were used to push the first molars mesially to develop an animal model of experimental tooth movement and to investigate the histologic changes of the periodontal ligament after tooth movement. In another study, this model of experimental tooth movement was also used to study the effect of inferior alveolar nerve transection on osteoclast numbers in periodontal ligaments during tooth movement. In our previous studies, we found an unmatched occlusa contact pattern between the maxillary and mandibular molars in the same animal model. Therefore, in this study, we adopted this animal model to investigate the influence of experimental tooth movement and occlusal changes on the myelinated nerve in the periodontal ligament.
Material and methods
All experimental procedures involving animals were conducted according to the Institutional Animal Care guidelines and were approved by the Administration Committee of Experimental Animals in the Fourth Military Medical University, Xi’an, China. Seventy-two female Sprague-Dawley rats, 8 weeks of age, weighing 200 to 220 g, were provided by the Laboratory Animal Center of the university. All animals received a standardized diet, and their body weights were recorded throughout the study. The rats were divided into 2 main groups, experimental-I and experimental-II ( Fig 1 , A and B ). Each subgroup was redivided into 3 time subgroups and a control group for immunohistochemistry (n = 5) and electron microscopic (n = 4) analysis, respectively. The detailed animal numbers are given in Table I . These rats were sexually mature at about 7 weeks of age, and their functional occlusion was established at about 5 weeks. Therefore, these rats were young adults with a stable functional occlusion.
| Main group | Subgroup | Control | 3 days | 14 days | 28 days |
|---|---|---|---|---|---|
| Experimental-I | For immunohistochemistry | 5 | 5 | 5 | 5 |
| For electron microscopy | 4 | 4 | 4 | 4 | |
| Experimental-II | For immunohistochemistry | 5 | 5 | 5 | 5 |
| For electron microscopy | 4 | 4 | 4 | 4 |
For the rats in the experimental-I group, 2 third molars were orthodontically moved distally as described previously. In brief, the rats were anesthetized with intraperitoneal injection of 1% pentobarbital (40 mg/kg). An elastic rubber band (3/16#, 1 mm in diameter, 2 mm in length; 3M Unitek, Monrovia, Calif) was stretched and inserted between the crowns of the maxillary left second and third molars, and also between the mandibular right second and third molars. We made sure that the bands did not reach the occlusal level of the molars and were short enough not to hamper the function of the adjacent soft tissues. Pushed by the elastic force of the rubber band, the crowns of the third molars gradually moved distally, creating a 0.8-mm-wide gap between the second and third molars and an unmatched occlusion between the maxillary and mandibular third molars ( Fig 1 , C and D ). One week later, the rubber band was replaced by self-curing resin to maintain the gap.
For the rats in the experimental-II group, after the anesthesia process, the mandibular left and right third molars were extracted before the elastic bands were inserted as for those in experimental-I group ( Fig 1 , E and F ). No procedure was performed on the control animals.
At 3, 14, and 28 days after the insertion of the rubber bands, the rats in both experimental-I and experimental-II groups were killed under deep anesthesia with 1.0% pentobarbital (40 mg/kg). The control animals were killed in the same way. All animals were fixed by transcarotid perfusion of 4.0% paraformaldehyde in 0.1 mol/L of phosphate-buffered saline solution (pH, 7.4). The animals’ maxillary jaws were cleaned, and the 2 alveolar bone sections that included separately the maxillary left and right third molars were sampled.
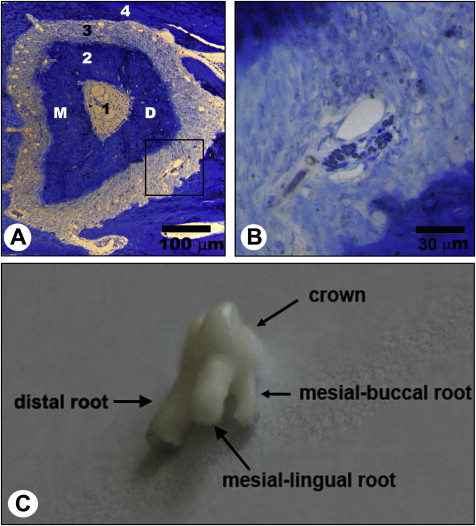
The third molars were further fixed with 3% glutaraldehyde in 0.1 mol/L of phosphate-buffered saline solution (pH, 7.4) at 4°C for 12 hours, decalcified with 5% EDTA-2Na (ethylene diamine tetraacetic acid disodium) solution for 4 to 5 weeks at room temperature. The distal roots of the maxillary third molars at both the left and right sides were dissected out of the maxillary jaws under a dissecting microscope. Specimens of the distal roots of the maxillary third molars were postfixed in 1% osmium tetroxide in 0.1 mol/L of sodium cacodylate buffer (pH, 7.3), dehydrated in ethanol, embedded in resin, and transversely prepared into semi-thin sections (thickness, 1.0 μm). The semi-thin sections were stained with a solution of 1% toluidine blue and 1% borax prepared in distilled water and examined under a light microscope (DM 2500; Leica, Wetzlar, Germany). The embedded specimens were trimmed accordingly to image the semi-thin sections, to confine the observation area to the distal half of the distal roots of the maxillary third molars where the myelinated nerve fibers were most frequently distributed ( Fig 2 , A and B ). The trimmed specimens were prepared into ultrathin sections (thickness, 50.0 nm). The sections were then stained with uranyl acetate and lead citrate and were examined under a transmission electron microscope (H-600; Hitachi, Tokyo, Japan).
Five samples at each time point were postfixed in 4.0% paraformaldehyde in 0.1 mol/L of phosphate-buffered saline solution (pH, 7.4) at 4°C for 12 hours, decalcified with Krinstenses’ solution (containing sodium formate and formic acid) for 2 weeks, dehydrated in ethanol, and fully immersed in soft paraffin. The samples were then carefully trimmed under the anatomic microscope, so that the roots of the third molars could be clearly seen and the starting plane of the consecutive slices could be accurately determined. The trimmed samples were embedded in paraffin and sectioned perpendicularly to the long axes of the molars. Consecutive slices, 5.0-μm thick, were sampled for immunohistochemistry.
Immunohistochemical staining was done with a 3-step avidin-biotin complex method. Briefly, the slices were dewaxed in xylene and dehydrated in an ethanol series. The endogenous peroxidase activity was eliminated by treating with 3% hydrogen peroxide. Nonspecific immunoreactivity in the slices was blocked by preincubation in 2% normal goat serum. The slices were then incubated overnight at 4°C with the primary antibody, a mouse antibody against myelin basic protein (1:50; Santa Cruz Biotechol, Santa Cruz, Calif). After incubation, the slices were then reacted with biotin-labelled IgG (BeiJing ZhongShanGolden Bridge Biotechnology, Beijing, China) at 37°C for 30 minutes and then with an avidin-peroxidase complex at 37°C for 30 minutes. Then a peroxidase/diaminobenzidine yellow kit (Wuhan Boster Biological Technology, Wuhan, China) was used for antibody staining. The slices were counterstained with hematoxylin and then dehydrated in an ethanol series, cleared in xylene, and cover-slipped. For the immunohistochemical control, the primary antibody was replaced by the phosphate-buffered saline solution. The slices were then cover-slipped with neutral balsam.
Immunohistochemically stained sections were observed under the light microscope. The distal roots of the bilateral maxillary third molars were selected for quantitative analysis. This was because the distal root is the largest root of the maxillary third molar ( Fig 2 , C ). The investigated plane was located at the apical third of the root, about 0.3 mm to the distal root apex.
Images of the observed area were acquired (50 times magnification, DFC490 system; Leica). For quantification of myelin basic protein in the periodontal ligament, the total area of the periodontal ligament of the root (total area) and the myelin basic protein positive area in the periodontal ligament were measured by using Image-Pro Plus software (Media Cybernetics, Rockville, Md). The ratio of the myelin basic protein positive area was calculated according to the following formula: ratio of myelin basic protein positive area (%) = (myelin basic protein positive area/total area) × 100%.
Statistical analysis
The average value of the 5 animals in each time point was derived, and the data were expressed as means and standard deviations. The statistical significance of the differences among the groups was evaluated by 1-way analysis of variance (ANOVA). If there was a significant overall difference among the groups, then the Tukey post-hoc test was used to make pair-wise comparisons. All statistical analyses were performed with SPSS software (version 12.0 for Windows; SPSS, Chicago, Ill). The level of significance was set at P <0.05 for all statistical tests.
Results
In the control group, the myelinated nerve fiber in the periodontal ligament was composed of the axon, the myelin sheath, the cytoplasm of the Schwann cell, and the basal membrane. The axon was filled with evenly distributed neurofilaments and mitochondria. The axon was encapsulated closely by the lamellated myelin sheath. The myelin sheath was mainly intact and continuous, with several artificial exfoliations. The axon-Schwann’s membrane, which consisted of the innermost Schwann’s membrane, the axon membrane, and a narrow gap between the membranes, could be clearly seen and was distributed evenly and consecutively. The cytoplasm of the Schwann cell was distributed outside the myelin sheath. The surface membrane of the Schwann cell was closely covered by a thin layer of basal membrane ( Fig 3 ).
In experimental-I group, in the maxillary left third molar with an opposing molar, degenerative ultrastructural changes were typically observed in the myelinated nerve, such as curved and incompact myelin lamellae. The changes were severest at day 3 and almost recovered to normal at day 28 ( Fig 4 , A-C ).
At day 3, no mitochondrion was observed in the axon. Curved and incompact myelin lamellae intruded into the axon, resulting in compressed neurofilaments with a high density in the axon, as well as discontinuous axon-Schwann’s membrane. In addition, some compact myelin lamellae were observed to protrude into the cytoplasm of the Schwann cell. Myelin globules (the disintegrated myelin sheath) and degenerated organelles were noted in the cytoplasm of the Schwann cell. The surface membrane of the Schwann cell and the basal membrane were continuous and intact ( Fig 4 , A ).
At day 14, the ultrastructural changes of nerve fibers were fewer compared with those at day 3. No mitochondrion was observed in the axon. Incompact myelin lamellae were found to intrude into the axon, but this change was slighter than that at day 3, resulting in relatively well-distributed neurofilaments in the axon. In addition, a vacuole-like structure and a compressed myelin lamellae sheath were observed in some regions of the myelin. The axon-Schwann’s membrane, the cytoplasm of the Schwann cell, the surface membranes of the Schwann cells, and the basal membranes were relatively normal ( Fig 4 , B ).
At day 28, a degenerated mitochondrion with a vacuole-like structure was observed in the axon. The degree of myelin sheath noncompaction was less than that at day 14. The neurofilaments were distributed evenly in the axon. The ultrastructures of the axon-Schwann’s membrane, the cytoplasm of the Schwann cell, the surface membrane of the Schwann cell, and the basal membrane were relatively normal ( Fig 4 , C ).
The pattern of ultrastructural changes of myelinated nerve in the maxillary right third molar with an opposing molar was similar to those in the maxillary left third molar with an opposing molar. However, the changes were smaller and found at later times compared with those in the maxillary left third molar with an opposing molar. The ultrastructural changes in the maxillary right third molar recovered to normal by day 28 ( Fig 4 , D-F ).
At day 3, localized high densities of mitochondria and neurofilaments were found in some regions in the axon. Myelin lamellae were incompact in several regions of the myelin sheath, without intruding into the axon or protruding into the cytoplasm of the Schwann cell. The axon-Schwann’s membrane, the cytoplasm of the Schwann cell, the surface membrane of the Schwann cell, and the basal membrane were relatively normal ( Fig 4 , D ).
At day 14, a degenerated mitochondrion and a myelin globule were observed in the axon. The myelin lamellae were incompact. The neurofilaments were distributed evenly in the axon. Degenerated membrane structures were found in the cytoplasm of the Schwann cell. The axon-Schwann’s membrane, the surface membranes of Schwann cells, and the basal membranes were intact and continuous ( Fig 4 , E ).
At day 28, almost normal structures were observed in most nerve fibers. In the remaining nerve fibers, myelin lamellae were incompact in a few regions of the myelin sheath. The neurofilament, the axon-Schwann’s membrane, the thickness of the myelin sheath, the surface membranes of Schwann cells, and the basal membranes of this nerve fiber were relatively normal ( Fig 4 , F ).
In the experimental-II group, in the maxillary left third molar with no opposing molar, ultrastructural changes of the myelinated nerves, characterized by shrunk axons, were typically observed. The degeneration of the myelin lamellae was not as obvious as that in experimental-I group. The changes in the maxillary left third molar with no opposing molar were severest at day 14 and did not recover to normal by day 28 ( Fig 5 , A-C ).
At day 3, a degenerated mitochondrion was observed in the axon. The mitochondria were distributed eccentrically along the axon membrane. Incompact myelin lamellae were found in several regions of the myelin sheath. The axon-Schwann’s membrane, the cytoplasm of the Schwann cell, the surface membrane of the Schwann cell, and the basal membrane were relatively normal ( Fig 5 , A ).
At day 14, the mitochondria were fewer than at day 3. Several myelin globules were found in the axon. Shrunk axons were observed, resulting in a high density of compressed neurofilaments in the axon and several gaps with low electron density between the axon membrane and the innermost Schwann’s membrane. The myelin sheath was mainly even and continuous. Degenerated membrane structures were observed in the cytoplasm of the Schwann cell. The surface membranes of the Schwann cells and the basal membranes were intact and continuous ( Fig 5 , B ).
At day 28, many mitochondria were observed in the axon. Shrunk axons were also observed, leaving several gaps with low electron density between the axon membrane and the innermost Schwann’s membrane. The myelin sheath was mainly even and continuous. The surface membranes of Schwann cells and the basal membranes of the nerve fibers were relatively normal ( Fig 5 , C ).
The ultrastructural changes of the myelinated nerve in the maxillary right third molar with no opposing molar were similar to those in the maxillary left third molar with no opposing molar, which was also characterized by shrunk axons. In addition, the changes were severest at day 14 and did not recover to normal by day 28.
At day 3, the mitochondria were distributed evenly in the axon. Slightly incompact myelin lamellae were observed in several regions of the myelin sheath. Degenerated membrane structures were found in the cytoplasm of the Schwann cell. The axon-Schwann’s membrane, the surface membrane of the Schwann cell, and the basal membrane were relatively normal ( Fig 5 , D ).
At day 14, the mitochondria were fewer than at day 3. Shrunk axons with compressed neurofilaments were also observed, resulting in many gaps with low electron density between the axon membrane and the innermost Schwann’s membrane. Myelin globules were observed in the axon. The surface membrane of the Schwann cell and the basal membranes were mainly normal ( Fig 5 , E ).
At day 28, mitochondria were found in the axon. The degree of shrinkage in the axons was less than at day 14. Gaps with low electron density between the axon membrane and the innermost Schwann’s membrane were also noted. The thickness of the myelin sheath, the surface membranes of Schwann cells, and the basal membranes of this nerve fiber were relatively normal ( Fig 5 , F ).
By immunohistochemistry, myelin basic protein positive structures could be detected. A cluster of myelin basic protein positive structures ( Fig 6 , A ), a bundle of myelin basic protein positive structures ( Fig 6 , B ), and scattered myelin basic protein positive structures ( Fig 6 , A-C, short arrows ) were detected.




