Introduction
Botulinum neurotoxins are responsible for the paralytic food poisoning, botulism. Commercial formulations such as botulinum neurotoxin type A are increasingly used for various conditions, including cosmetic recontouring of the lower face by injection of the large masseter muscles. The paralysis of a major muscle of mastication lowers occlusal force and thus might affect tooth eruption. The purpose of this study was to investigate the effects of unilateral masseter muscle injection of botulinum neurotoxin type A on the rate of eruption of incisors in a rabbit model. We hypothesized that the teeth would overerupt in an underloaded environment.
Methods
Forty rabbits were injected with either botulinum neurotoxin type A or saline solution in 1 masseter muscle. Mastication and muscle force production were monitored, and incisor eruption rate was assessed by caliper measurement of grooved teeth.
Results
The injection of saline solution had no effect. The masseter muscle injected with botulinum neurotoxin type A showed a dramatic loss of force 3 weeks after injection despite apparently normal mastication. Incisor eruption rate was significantly decreased for the botulinum neurotoxin type A group, an effect attributed to decreased attrition.
Conclusions
This study has implications for orthodontics. Although findings from ever-growing rabbit incisors cannot be extrapolated to human teeth, it is clear that botulinum neurotoxin type A caused a decrease in bite force that could influence dental eruption.
Botulinum neurotoxins, produced by anaerobic Clostridium bacteria, are responsible for botulism, a paralytic food poison. These proteases interfere with the vesicle fusion apparatus, thus blocking the release of acetylcholine at neuromuscular junctions. In 1989, botulinum neurotoxin type A serotype (BoNT/A) was approved by the FDA for treating blepharospasm. As a side effect of its use on muscles of the face, it was noted that it reduced facial wrinkles, leading to its popularity in cosmetic enhancement. At therapeutic dosages, BoNT/A has a wide safety margin, leading many clinicians to use it off label.
One off-label use of BoNT/A is in jaw muscles. BoNT/A is currently used for the cosmetic reduction of large masseter muscles and in treating temporomandibular joint disorders, dystonias, and migraine headaches. Even though published studies on the subject are generally “of low quality (noncomparative, nonrandomized trials),” the efficacy of the toxin for use in muscle spasms and cosmetic reduction is well established. If an adequate dosage is used, muscle shrinkage occurs reliably, but not always evenly, as the denervated fibers undergo atrophy and dissolution.
The clinical literature on BoNT/A in the masseter muscle indicates that maximal atrophy of the masseter follows loss of electromyography by about 2 months and is still sometimes observable at 1 to 2 years. Occlusal force is related to muscle activity and size. Yet these parameters do not covary after BoNT/A. In 1 report, voluntary bite force was subjectively normal in 8 days even though symptom relief persisted for 8 weeks. In the best documented study to date, bite force began to recover in week 3 when muscle volume was still decreasing; bite force was fully recovered at 3 months, when the muscle was at minimal volume. It is not clear how an atrophied muscle can produce a normal bite force, but a possible explanation is that the subjects had simply learned to produce normal voluntary force by using untreated muscles.
The presumed reduction of functional occlusal force after BoNT/A has consequences for the dentition, even if the loss is brief. The forces of occlusion are an important factor inhibiting the eruption of teeth. The primary evidence for this inhibition comes from loss of occlusion. When an opposing tooth is removed experimentally or clinically, eruption speeds up, and overeruption occurs. Muscle weakness in humans and muscle resection in rats have the same effect. Because BoNT/A paralysis also causes muscle weakness, it can lead to supraeruption. At the same time, however, the loss of occlusal force would decrease the amount of attrition on the tooth crowns, and this effect might actually slow eruption, at least in rapidly worn, continuously erupting teeth such as rodent and rabbit incisors. Eruption rate has not been studied systematically for BoNT/A treatment in either human or animal models. Control of the vertical dimension of occlusion is an important consideration for orthodontists in treating patients. Weak masticatory musculature, decreased occlusal force, and a tendency for extrusion have been associated with malocclusions characterized by increased anterior facial height and high mandibular plane angles. Whereas the use of BoNT/A in these patients might exacerbate this extrusive tendency, it can prove useful in patients with a decreased lower facial height and large musculature, in which extrusion would be beneficial but difficult because of their strong biting forces.
Continuously growing teeth, such as rabbit and rat incisors, have a stable alveolar crest, while the tooth-forming base remains in the same position in the socket. The rapidity of eruption of rabbit incisors (10 times faster than human eruption) has made it a favorite study model. For this reason, rabbits were chosen for this study, the purpose of which was to investigate the effects of unilateral masseter muscle injection of BoNT/A on the rate of tooth eruption. We expected that this loss of force would increase the rate of eruption of the incisors.
Material and methods
We used rabbits from a previous study. In brief, 40 female New Zealand white rabbits (Western Oregon Rabbit Company, Philomath, Ore) were obtained in groups of 8 at a time, except for 1 group of 9 because a rabbit expired prematurely in the previous group. This resulted in a sample of 21 animals treated with BoNT/A and 19 treated with saline solution, rather than 20 and 20 as originally planned. The sample size was based on a power analysis indicating that 20 animals per group would yield 95% power to detect a 1.7 effect-size difference in bite force with α = 0.05. The rabbits were 5 months of age and weighed between 3.8 and 5.1 kg at the beginning of the study. They were fed only rabbit pellets (Rabbit 16%; Albers Animal Feed, Portland, Ore). The Institutional Animal Care and Use Committee at the University of Washington approved our protocol.
The rabbits were acclimated to feeding in the laboratory environment; they were placed and fed in an acrylic plastic holding box for approximately 1 to 2 hours per day for 1 to 2 weeks. They received 8 oz of pellets per day from a food tray attached to the front of the holding box. For the rest of the time, they were housed in the animal facility without food except for the weekends. If the rabbits did not finish the 8 oz of food provided during the laboratory session, the rest was given in their cages.
After the initial acclimatization period, the animals were randomized to receive either BoNT/A or saline solution injections by coin toss. The investigators were blinded to treatment. BoNT/A (Botox Cosmetic; Allergan, Irvine, Calif) was reconstituted according to the package insert (100 units of toxin in 2.5 mL 0.9% of sterile saline solution). The animals were shaved to expose the skin over the masseter muscle, the forehead, and the submandibular area ( Fig 1 ). Each animal received injections of either BoNT/A or saline solution in 1 masseter muscle, chosen by coin toss. The volume injected was 0.25 mL of BoNT/A (10 units) or saline solution, divided into thirds and injected into 3 target areas paralleling the lower border of the angle of the mandible into the middle thickness of the muscle, with injection continuing as the needle was withdrawn. These locations were chosen because they approximated the location of the motor end plates. The injection sites were massaged for a few minutes to encourage dispersion of the fluid as per clinical usage.
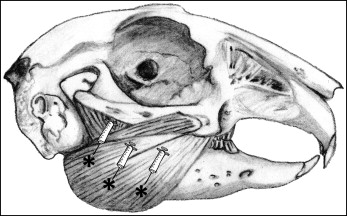
This study was carried out during week 3 (days 15-22 after the injections), when incisor bite force was expected to be minimal. The investigators were blinded as to treatment group and side of treatment. The animals were weighed before treatment and several times per week after treatment with a digital scale (model 6745; Detecto, Webb City, Mo).
Electromyographic readings and simultaneous videos were recorded weekly while the animals were allowed to eat in their holding boxes. These data, previously published, indicated that injection of saline solution had no effect on muscle activity, nor was there any change in the activity levels of the noninjected masseter muscles of the BoNT/A animals. However, the effect of BoNT/A injection on masseter activity was profound. Electromyographic values were reduced by 40% to 70% compared with pretreatment values 1 to 2 weeks after the injection.
As an index of masticatory efficiency during week 3, a random subset of 10 rabbits injected with saline solution and 10 injected with BoNT/A were individually recorded consuming a measured volume of food. The number of cycles required to masticate a specified amount of food was determined by putting a bolus of pellets (approximately 1 g) into the rabbit’s mouth with a 1-mL insulin syringe with the needle portion removed (Becton Dickinson, Franklin Lakes, NJ). The bolus was weighed on a scientific scale (PC 400; Mettler, Columbus, Ohio) accurate to 0.01 g. An effort was made to vary the side of the mouth for delivery, but a previous study demonstrated that the side of food insertion is independent of the chewing side in rabbits. Any pellet not consumed or that fell out of the mouth was collected and weighed. This was repeated 10 times for each rabbit, allowing sufficient time between to determine that all the food was consumed. Rabbits that did not voluntarily masticate the entire food bolus or held it in their cheeks were given another attempt later and excluded from this analysis if voluntary mastication was not observed. Approximately half of the rabbits did not completely chew the bolus, keeping the food in their cheeks, resulting in a final sample size of 6 saline solution and 5 BoNT/A rabbits. The delivery of the bolus and its subsequent mastication were recorded with a digital video camera. The number of chewing cycles for each bolus was counted on the video recording, and masticatory efficiency was calculated as the number of cycles per gram of food.
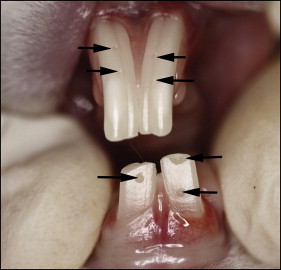
Incisor marking and assessment of tetanized muscle force were performed with the rabbits under isoflurane anesthesia (Narkovet 2; North American Drager, Telford, Pa) with a custom-built nasal mask. Rabbits have 2 maxillary incisors per quadrant: a large anterior tooth and a small posterior one behind the anterior incisor. Together, the maxillary incisors form a notch into which the mandibular incisor fits. The labial surfaces of the anterior maxillary incisors and the mandibular incisors were marked at the level of the free gingival margin in the midline of each tooth with a small-diameter bur (FG330; SS White, Lakewood Township, NJ) in an air-powered, high-speed dental hand piece (model 430K; Star Dental, Lancaster, Pa). This shallow reference groove did not penetrate the enamel layer ( Fig 2 ). If any space remained between the apical margin of the reference groove and the most apical point of the free gingival margin, it was recorded. At weekly to biweekly intervals, the space between the most apical point of the free gingival margin and the most apical margin of the reference groove was measured with digital calipers. Incisor eruption measurements were initially taken while the animals were anesthetized; it was later possible, once the animals were acclimatized to the laboratory environment, to do it without anesthesia, simply by retracting their lips manually. As the marker reached the incisal edge, new reference grooves were placed. This resulted in most teeth having 2 grooves available for measurement on any given day. Both were measured, providing an assessment of repeatability. These paired measurements yielded essentially identical readings. The groove technique was adopted after an attempt to mark the incisors with a bonded dental composite was abandoned because the rabbits wore off the composite. Attempts were also made to mark the molars, but access issues made accurate placement and measurement impossible. It was also impossible to track the posterior maxillary incisor, but it was assumed that its behavior mimicked that of the anterior maxillary incisor.
Bite force was measured at the incisors in anesthetized animals while each masseter muscle was separately tetanized. Wire electromyographic electrodes, situated anteriorly and posteriorly in the masseter, were stimulated (Grass models S48 and SIU5; Astro-Med, West Warwick, RI; or the stimulator function of model M150; Biopac, Goleta, Calif) with trains of 5 msec pulses delivered at 55 Hz for 550 msec at a voltage determined to be supramaximal by gradually ramping up the voltage, with intervals of 5 to 10 seconds between trains. This procedure was necessary to determine maximal bite force but might have resulted in muscle fatigue. However, all muscles received the same stimulation protocol, and all investigators were blinded to the treatment of each muscle. Thus, although muscle fatigue might underestimate the absolute value of bite force, differences measured between muscles are nonetheless valid. The bite force transducer was based on the design of Dechow and Carlson and included a small groove for the maxillary incisors to ensure consistent placement. The transducer readings were converted to kg-force by using a previously determined calibration equation. The right and left masseter muscles were tetanized in random order. Repeat stimulations were not carried out to avoid fatiguing the muscles further.
Descriptive statistics were calculated for each variable. Incisor eruption rates were analyzed for each tooth and compared between the 2 groups. The primary hypothesis was that eruption would be faster in animals injected with BoNT/A than in those injected with saline solution. This was assessed as a 2-sample t test of the null hypothesis of no difference between the groups, performed for each tooth position. Because the incisors function as a unit, no difference was expected between eruption of the teeth on the injection side vs the noninjection side. This null hypothesis was tested within groups by using paired t test comparisons. The significance level was set at P <0.05. The statistical analysis was accomplished by using SPSS software (version 17.0; SPSS, Chicago, Ill). Power calculations were carried out with G*Power.
Results
Overall, the rabbits tolerated the experiments well. Some data were missing, primarily because of equipment malfunction, and this is reflected in the varying sample sizes. There was no initial difference between the BoNT/A and saline solution groups in any parameter, including body weight, electromyographic activity levels, masticatory performance, or stimulated masseter incisor bite force.
The Table compares these variables at week 3 postinjection for the 2 groups and shows that body weight and masticatory parameters still did not differ significantly. The BoNT/A animals were actually slightly heavier and chewed slightly more on the injected side than did the animals injected with saline solution. However, bite force produced by tetanus of the muscles injected with BoNT/A had plummeted compared with that produced by tetanus of the muscles injected with saline solution ( P <0.0001). The noninjected side was unaffected.
| BoNT/A | Saline solution | P value | |
|---|---|---|---|
| Body weight (kg) | 4.04 ± 0.27, 21 | 3.95 ± 0.36, 19 | 0.40 |
| Mastication | |||
| Rate (Hz) | 3.3 ± 0.33, 21 | 3.41 ± 0.29, 19 | 0.52 |
| Side (% on injected side) | 58 ± 24, 21 | 49 ± 29, 19 | 0.52 |
| Efficiency (cycles/1-g bolus) | 126 ± 39, 5 | 117 ± 20, 6 | 0.65 |
| Bite force from tetanized masseter (kg) | |||
| Noninjected side | 0.98 ± 0.68, 18 | 1.04 ± 0.43, 18 | 0.40 |
| Injected side | 0.22 ± 0.21, 16 | 1.38 ± 0.90, 16 | 0.00 ∗ |
| P value between sides | 0.00 ∗ | 0.16 | |
| Incisor eruption rate (mm/day) | |||
| Maxillary noninjected side | 0.24 ± 0.09, 16 | 0.34 ± 0.11, 16 | 0.01 ∗ |
| Maxillary injected side | 0.24 ± 0.09, 16 | 0.32 ± 0.09, 16 | 0.02 ∗ |
| Paired P value between sides | 0.81 | 0.45 | |
| Mandibular noninjected side | 0.38 ± 0.14, 16 | 0.49 ± 0.12, 16 | 0.02 ∗ |
| Mandibular injected side | 0.32 ± 0.14, 16 | 0.46 ± 0.16, 16 | 0.01 ∗ |
| Paired P value between sides | 0.06 | 0.45 |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


