Fig. 11.1
Facial hematoma following endodontic surgery of the mandibular premolar
Ecchymosis is an extravasation of blood into subcutaneous tissue or mucosa (Fig. 11.2) [11–13].
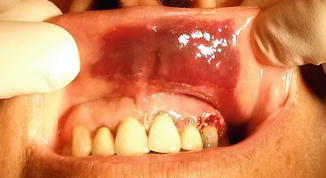

Fig. 11.2
Ecchymosis of the oral mucosa at the upper lip region following endodontic surgery of the maxillary incisors
Ecchymosis or mucosal petechiae are quite unpredictable. The inflammatory reaction after surgery and subsequent fragility of the capillaries may be a major contributor to ecchymosis and petechiae development [11]. Although both are transient symptoms, skin ecchymosis is unaesthetic. Therefore, its prevention is useful [12]. Skin ecchymosis can be induced by a problem in the production of coagulation factors by the liver, inadequate reticuloendothelial clearance of fibrin degradation products, and decreased production of platelets, which promote secondary fibrinolysis [13]. Ecchymosis can last for up to 2 weeks presenting an esthetic problem and generally requires no treatment.
Mild hemorrhage is relatively common during endodontic surgery, and although it is usually not life threatening, it may cause complications during the surgical procedure and may even jeopardize the prognosis of the treatment [4]. Adequate bleeding control is essential for the success of periapical surgery, since it improves visualization of the surgical site, minimizes the operating time, and enables the dry field for retrograde filling material placement [4, 7].
This chapter will discuss the local and systemic considerations of the prevention, diagnosis, and management of bleeding-related complications in endodontic surgery.
Systemic Considerations
Hemostasis may be defined as the process whereby bleeding is controlled [14]. Biologically, hemostasis is a tightly regulated process that maintains the blood flow through the vasculature simultaneously as a thrombotic response to tissue damage occurs [6, 7]. The hemostatic process is extremely complex and for simplicity may be classified to a primary phase, initiated at time of injury and ends with the formation of an unstable soft plug, and an ensuing secondary phase that starts by the initiation of the full-scale coagulation cascade that ends in the formation of a stable fibrin clot [6, 7]. Impairment in one or more of the hemostasis cascade components may lead to bleeding disorder and an increased potential for bleeding-related complications during surgery [3–7, 15, 16].
Bleeding during or following minor surgical procedures of the oral cavity is not rare and fortunately, in healthy patients, is usually self-limiting and manageable [3]. However, a small sector of the general population has an increased risk of bleeding due to inherited bleeding disorders, and even more common are patients with hemostatic impairments that are secondary to underlying disease or medication [3]. In those population groups, even a relatively minor surgical procedure may precipitate an excessive and prolonged bleeding incident [3], impaired wound healing, and increase risk of infection [3]. It has been reported that while the incidence of postoperative bleeding following minor oral surgical procedures is within the range of 0.2 and 3.3 %, in patients under chronic anticoagulation therapy, the occurrence of postoperative bleedings is much more prevalent and ranges between 8.6 and 32.1 % [5].
Identification of patients with bleeding disorder is a key objective of the preoperative anamnesis and evaluation, in order to prevent surgical complications. The practitioner needs to be familiar with the different categories of bleeding disorders, their clinical manifestation, and most importantly, their clinical relevance to the planned surgical procedure [3, 5–7]. In general, bleeding disorders may be divided for simplicity to platelets disorders, inherited coagulation disorders, and acquired coagulation abnormalities.
Platelet Disorders
There is a number of platelet-related defects, both inherited and acquired, that may be grossly divided as defects in the number of platelets (i.e., “thrombocytopenia”) or of platelet function, though some platelet disorders are characterized by both decreased number and impaired platelet function [3, 5].
Normal blood platelet levels are usually within the range of 150–400 × 109/L, and thrombocytopenia leading to increased bleeding is rarely clinically significant unless platelet counts are less than 50 × 109/L [3, 5]. On the other hand, platelet function disorders, such as adhesion or aggregation defects, may lead to surgical bleeding and may require preparation prior to surgery, such as platelet transfusion [3, 5].
Inherited Coagulation Disorders
Von Willebrand disease (VWD) results from quantitative or qualitative defects in VW factor, a key protein in hemostasis, and is the most common inherited bleeding disorder, affecting up to 1 % of the general population [3]. The clinical manifestations of VWD are easy bruising, epistaxis, menorrhagia, and operative bleeding [3].
Hemophilia is an inherited bleeding disorder caused by deficiencies of either factor VIII (termed: “hemophilia A”) or factor IX (“hemophilia B”) [3, 5]; both types are not clinically distinguishable [3, 5]. The prevalence of hemophilia is 1 in 5,000 males [3, 5]. Hemophilia patients are characterized by easy bruising, excessive post-trauma bleeding, spontaneous muscle and joint hemorrhage, and excessive bleeding following surgical procedures [3, 5].
Additional congenital coagulation deficiencies are extremely rare [3, 5]. Thus, although factor XI deficiency in the Ashkenazi Jewish population has a prevalence of 1 in 1,000, the prevalence of other factors deficiencies is in the range of 1 in 0.5–1 per million [3, 5]. Usually, with possible some exceptions, the bleeding manifestations in these disorders are less severe than in hemophilia patients [3, 5].
Acquired Coagulation Abnormalities
Patients on chronic anticoagulation therapy are at increased risk of bleeding during surgical procedures [3, 5], and the risk of bleeding is relative to the intensity and duration of the anticoagulation therapy [3, 5].
Warfarin is a relatively common anticoagulation agent indicated usually for prevention of thromboembolism [3, 5]. Warfarin is a vitamin K antagonist, and its effect is monitored by the international normalized ratio (INR; a standardization of the prothrombin time assay). The therapeutic INR may vary depending on the clinical indication, but is usually within the range of 2.0–3.0 for most patients [3, 5].
The management of patients who are receiving warfarin and require endodontic surgery is a relatively common clinical difficulty, and when indicated, warfarin treatment interruption is clinically simple since it requires just waiting until the anticoagulant effect wears off and resume it when there is adequate hemostasis. However, it requires a complex decision because of thromboembolic risks during anticoagulant interruption [17]. Case selection of patients on warfarin is therefore very important, and patients with coexisting medical problems (such as liver disease, renal disease, and thrombocytopenia or who are taking antiplatelet drugs) in certain cases should NOT have a surgical dental procedure in the primary care setting [18].
The risk of significant bleeding in patients on oral warfarin with stable INR levels in the therapeutic range is low. However, the risk of thrombosis if warfarin treatment is discontinued may be increased. Thus, oral anticoagulants should not be routinely discontinued in patients requiring endodontic surgery, and the matter should be discussed with the patient’s hematologist, particularly when the INR levels are high or when the INR levels are unstable [18].
In patients receiving warfarin, an INR check shortly prior to surgery is recommended. Perry et al. [18] recommended that in patients receiving long-term warfarin and who are stably anticoagulated, an INR check 72 h prior to surgery is recommended to allow sufficient time for dose modification if necessary to ensure a safe INR on the day of surgery [18]. Douketis [17] recommended an INR check 1 day prior to an elective surgery.
It is important to note that the commonly used NSAIDs should be avoided in patients receiving warfarin because of their antiplatelet action and the risk of over anticoagulation and hemorrhage [17].
When the risk for bleeding during or following the surgery is significant, in certain cases warfarin interruption is indicated. In addition, for patients with significant risk of thromboembolism following warfarin interruption, anticoagulation bridging may be required [17]. Bridging anticoagulation for warfarin interruption consists of warfarin stop about a week prior to surgery and start of heparin bridging [17]. The decision if and how to interrupt an anticoagulation treatment and whether to adopt an anticoagulation bridging protocol is a complex decision that requires consultation with the patient’s primary physician [17, 18].
Heparin is a cofactor of the naturally occurring anticoagulant antithrombin, accelerating inhibition of the serine proteases of the coagulation cascade [3, 5], and has a short half-life (about 1 h, for “unfractionated” heparin) [3, 5]. Heparin is given by intravenous bolus followed by infusion to maintain its therapeutic levels. However, low molecular weight heparin (LMWH) possesses a longer half-life than unfractionated heparin and can be delivered daily subcutaneously [3, 5]. Usually, most patients on long-term heparin therapy do not require laboratory monitoring. However, when monitoring is indicated, an anti-Xa assay is used [3, 5].
It is important to note that sometimes the preoperative anamnesis and the routine clinical evaluation may not reveal an underlying clinically significant bleeding disorder [5]. Thus, in case of unexplained prolonged and difficult-to-manage intraoperative bleeding or in case of recurrent postoperative bleedings, the surgeon should always consider the possibility of an undetected underlying systemic bleeding disorder [3, 5, 17, 18].
The possibility of intra- or postoperative bleeding always exists when a surgical procedure is undertaken, especially in patients receiving anticoagulation therapy or with an underlying systemic bleeding disorder [3, 5, 17, 18]. A thorough preoperative evaluation and anamnesis are needed in order to screen for potential bleeding disorders. Before surgery, and especially in patients with systemic bleeding disorders, the practitioner is required to ask himself or herself several key questions before he or she performs the surgical procedure [3, 5, 17, 18]:
-
What is the exact bleeding disorder, including the severity of the disorder, its current and updated status, and its clinical relevance to the planned surgical procedure?
-
Do I have all necessary means (including knowledge, clinical settings, and equipment) to manage any potential bleeding during or following the surgical procedure?
-
Is the potential benefit to my patient outweighs the potential risks associated with bleeding?
Local Considerations
Achieving proper hemostasis is an essential principle of surgery, and achieving adequate hemostasis in bone is particularly important during endodontic surgery [19].
Prevention and management of bleeding during surgery is a complex and multilayered process that includes preoperative, intraoperative, and postoperative considerations [7].
The surgeon’s actions play a significant role in achieving surgical hemostasis [6, 7, 16]. Thus, proper preoperative evaluation, integration of relevant anatomical and systemic consideration into the treatment planning, and most importantly adequate surgical procedures are the key for achieving appropriate bleeding control during surgery [7].
Anatomical Considerations
It is common to speculate that different arteries supply certain specific regions of the periodontium and of the dentition. However, in fact, there are abundant anastomoses present between the different arteries. Thus, the entire system of blood vessels, rather than a specific group of vessels, should be regarded as the supplying source of the soft and hard tissue of the jaws [20–23].
The anatomy of the major blood vessels of the maxillofacial region is relevant to the risk of severe hemorrhage and massive hematomas [5, 7, 8, 10]. Thus, the treatment planning and all the surgical procedures, such as flap design and osteotomy, should respect the anatomical structure of the blood perfusion system of the periodontium, in order to minimize potential complications such as surgical bleeding.
Mandible
Anastomoses of the sublingual and submental arteries are responsible for the arterial blood supply of the floor of the mouth. The submental artery is a branch of the facial artery. The sublingual artery (2 mm in average diameter) arises from the lingual artery and is found coronal to the mylohyoid muscle [20
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


