Implants are exposed to a diverse oral environment and host responses that contribute to health or disease. For the last few decades, clinicians have relied on standard clinical and radiographic findings to assess the health of implants. However, recent studies involving the pathogenesis of peri-implantitis have identified microbial species and several putative biomarkers that could aid clinicians in this diagnostic process in the near future. This article provides an overview of the microbial species involved in implant health and disease and biomarkers found in oral fluids that relate to the underlying biological phases of a failing implant.
Key points
- •
The diagnosis of peri-implantitis benefits from clinical, radiographic, microbiological, and biological information.
- •
Practitioners and patients can use biomarkers to identify risk of disease, disease activity, disease progression, and response to therapy.
- •
Peri-implantitis is a biofilm-induced condition. The microbial composition of peri-implantitis lesions is mixed, nonspecific, and less diverse than that of periodontitis but includes Fusobacterium, Prevotella, Porphyromonas, Streptococcus, Campylobacter , and Neisseria species.
- •
Failed implants are often associated with enteric bacteria, spirochetes, and opportunistic bacteria (ie, Staphylococcus aureus ).
- •
Protein biomarkers detected in peri-implant crevicular fluid provide insight into the underlying biology of the disease and specificity regarding the stage of the disease.
Introduction
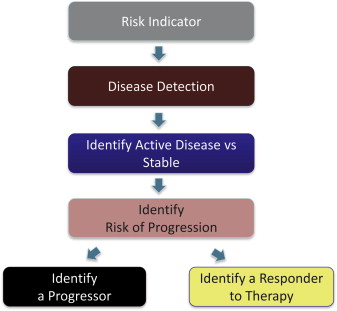
As a result of clinical translational research, biomarkers are becoming increasingly available. They supplement clinical and radiographic information, allowing clinicians to make better decisions. Patients can also use biomarkers to obtain information about their health status and the need for dental care. Although biomarkers are most commonly used to decide whether a patient has a disease, their usefulness is more expansive. As Fig. 1 shows, biomarkers are important for identifying severity of disease, ongoing activity of disease, disease progression, and response to therapy. With respect to periodontal disease, salivary analytes interleukin 1 beta (IL-1β), matrix metalloprotease 8 (MMP-8), and macrophage inflammatory protein-1 alpha (MIP-1α) have recently been shown to serve in these roles. For example, high salivary concentrations of these analytes are associated with periodontal disease, whereas high salivary concentration of MIP-1α is also a predictor of risk, that is, predictive of alveolar bone loss 6 to 9 months before radiographic evidence is apparent. Oral fluid biomarkers can also be used to indicate response to therapy and have recently been shown to be useful in this role. Together, the identification of biomarkers that have clinical utility for risk identification, disease detection, and identification of disease progression and response to therapy is the basis for establishing personalized care in the modern health care age and serve as the context for this article on peri-implantitis and failing dental implants. Specifically, this article discusses the milieu of microbes and proteins, that constitute the underlying biology of implant osseointegration and disease progression that can serve as indicators of implant health or failure.
Introduction
As a result of clinical translational research, biomarkers are becoming increasingly available. They supplement clinical and radiographic information, allowing clinicians to make better decisions. Patients can also use biomarkers to obtain information about their health status and the need for dental care. Although biomarkers are most commonly used to decide whether a patient has a disease, their usefulness is more expansive. As Fig. 1 shows, biomarkers are important for identifying severity of disease, ongoing activity of disease, disease progression, and response to therapy. With respect to periodontal disease, salivary analytes interleukin 1 beta (IL-1β), matrix metalloprotease 8 (MMP-8), and macrophage inflammatory protein-1 alpha (MIP-1α) have recently been shown to serve in these roles. For example, high salivary concentrations of these analytes are associated with periodontal disease, whereas high salivary concentration of MIP-1α is also a predictor of risk, that is, predictive of alveolar bone loss 6 to 9 months before radiographic evidence is apparent. Oral fluid biomarkers can also be used to indicate response to therapy and have recently been shown to be useful in this role. Together, the identification of biomarkers that have clinical utility for risk identification, disease detection, and identification of disease progression and response to therapy is the basis for establishing personalized care in the modern health care age and serve as the context for this article on peri-implantitis and failing dental implants. Specifically, this article discusses the milieu of microbes and proteins, that constitute the underlying biology of implant osseointegration and disease progression that can serve as indicators of implant health or failure.
Definitions
Peri-implantitis is a potentially progressive condition involving infection, inflammation, connective tissue destruction, and bone resorption. The condition is characterized by microbial infection, deep probing depths, bleeding on probing, suppuration, and radiographic bone loss. Risk factors include cigarette smoking, poor oral hygiene, and a previous history of periodontitis. Peri-implantitis does not necessarily mean that the implant will fail. The implant can be salvaged if peri-implantitis is diagnosed early, if risk factors are reduced or eliminated, and if the site is treated appropriately. In contrast, implant failure is defined as the inability of the host tissue to establish or maintain osseointegration, which is clinically diagnosed by mobility of the implant ( Fig. 2 ).
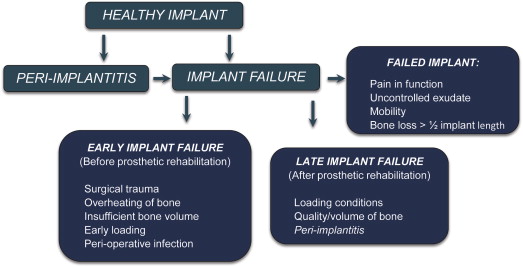
Implant failures are classified as early or late, depending on the time of placement and the implant’s functionality. Early failure occurs before prosthetic rehabilitation and before the implant is placed into function. Early failures generally result from surgical trauma, overheating of the bone during implant surgery, insufficient bone surrounding the implant, early loading of the implant, or perioperative bacterial infection. Late failure occurs after prosthetic rehabilitation and indicates that established osseointegration has not been maintained. Late failures can further be classified as early or delayed depending on whether implant failure is observed before or after the first year of loading. Delayed late failures are generally associated with changes in loading conditions, the quality and volume of bone relations, and peri-implantitis.
A failed implant is a clinical diagnosis defined by one or more of the following criteria: pain in function, presence of uncontrolled exudate, mobility, or radiographic alveolar bone loss more than half the length of the implant. Clinicians generally recommend removal of a failed implant ; however, because implant failure is a process requiring time, a clinician can experience the dilemma of a failing implant, characterized by progressive loss of alveolar bone support. In this instance, the clinician must decide what action to take and whether clinical, radiographic, or biological information can help in the decision-making process that could ultimately prevent the loss of the implant. Clinicians should be aware that a failing implant is associated with the accumulation of microbial plaque and bacterial infection around the implant. These microbes elicit many biological mediators, and it is conceivable that the milieu of microbial and biological molecules that surround and emanate from the sulcus of the implant can distinguish a healthy implant from one associated with peri-implantitis.
Microbiological markers of failing dental implants
Peri-implantitis accounts for 10% to 50% of implant failures after the first year of loading. Microorganisms play an important role in peri-implantitis; therefore, the identification of peri-implantitis-associated microbiota or bacteria is crucial for an understanding of peri-implantitis pathogenesis and of the bacteria that could serve as microbial biomarkers of this condition. Table 1 summarizes the results of studies conducted during the last 3 decades that have analyzed the microbiota of peri-implantitis sites. Details regarding these studies are discussed below.
| Authors | Follow-up | Implants (Patients) | Method | Results |
|---|---|---|---|---|
| Salvi et al, 2008 | 12 mo | 17 I 17 T (n = 13) |
DNA-H | Sum of bacterial counts at T sites was higher than at I sites. Pm, Lb, Cs, and Pi were most prevalent at I sites at 12 mo (>30 × 10 5 cells). Sg, Fn ssp , polymorphum, and vincentii were most prevalent at T sites at 12 mo (>50 × 10 5 cells). F spp , Strep spp , Pm, and Sa were higher at I sites. Few differences in bacterial species between tooth and implant at 12 mo. |
| Furst et al, 2007 | 3 mo | 17 I 17 T (n = 14) |
DNA-H | Bacterial colonization around implants starts at 30 min. Pg, Td, and Tf were present at implant sites at 12 wk. Sa was present at 15% of the implant sites at 12 wk. |
| Quirynen et al, 2006 | 18 mo | NR (n = 42) | DNA-H culture | Red (5%) and orange (20%) complex bacteria were present around the implant at 1 wk. Red (8%) and orange (33%) complex bacteria were present around the implant at 3 mo. The subgingival microbiota was similar at I and T sites at 3 mo. |
| Leonhardt et al, 2002 | 10 y | 57 I 261 T (n = 15) |
Culture | Pg, Pi, Aa, C ssp, and Cr were detected at baseline and at 10-yr follow-up at T and I sites. These bacterial species are members of the normal resident microbiota. |
| Hultin et al, 2000 | 10 y | 43 I 31 T (n = 15) |
DNA probe | No marked differences were found between T and I at 10 yr. Td, Si, and Pm were the most common bacteria at I and T sites. Aa, Pg, Td, and Tf were found at implant sites with > 2-mm bone loss. |
| van Winkelhoff et al, 2000 | 12 mo | NR (n = 20) | Culture | Prevalence of bacteria was similar at I and T sites at 6 mo. Implant failure was associated with high levels of Pg. Aa was not detectable around I sites. |
| Sbordone et al, 1999 | 3 y | 42 I 25 T (n = 25) |
Culture | Pg and C ssp were the most prevalent bacteria around implants at 1 yr. Significantly fewer motile rods were found at implant sites than at teeth at 1 yr. No statistically significant difference in periodontopathogens at implant and tooth sites. |
Bacterial Colonization and Microbial Composition Around Healthy Implants
Longitudinal studies of biofilm formation around dental implants have shown that bacterial colonization occurs immediately after implant placement (within 30 minutes). The microbiota associated with healthy peri-implant tissues are dominated by gram-positive facultative cocci and rods and by low proportions of gram-negative anaerobic rods. Initial colonization of peri-implant sites with periodontitis-associated bacteria (eg, Porphyromonas gingivalis, Treponema denticola, Tannerella forsythensis ) is detected as early as 2 weeks after placement. The composition of bacteria around healthy teeth and around healthy implant sites is reported to remain similar for as long as 2 years. However, the sum of bacterial counts of 40 periodontopathogenic species analyzed with DNA-DNA hybridization was higher around normal teeth than at implant sites both at baseline (immediately after implant placement) and 1 year later. Also, the most predominant microbes around implants 1 year after placement were Peptostreptococcus micros, Leptotrichia buccalis, Capnocytophaga sputigena , and Prevotella intermedia (>30 × 10 5 cells), whereas the most predominant microbes at tooth sites were Streptococcus goordinii and Fusobacterium nucleatum subspecies polymorphum, and vincentii (>50 × 10 5 cells). However, the presence of putative periodontal pathogens at peri-implant sites does not dictate loss or failure of an implant attachment, provided proper oral hygiene measures and periodontal supportive therapy are maintained. Two follow-up studies of clinical, radiographic, and microbiological parameters of osseointegrated implants in partially edentulous patients treated for periodontal disease did not report marked differences in microbial flora between healthy implants and teeth at baseline and 10 years later. In fact, putative bacterial species, including P gingivalis, P intermedia, Aggregatibacter actinomycetemcomitans , Capnocytophaga spp, and Campylobacter rectus , were present at clinically healthy implant sites at a 10-year examination. Therefore, it is suggested that periodontopathogens are present at implant sites as part of the normal resident microbiota and are not the sole factor affecting the long-term outcome of implant treatment.
Microbial Composition Around Implants Associated with Peri-implantitis
Today, it is well accepted that peri-implant mucositis and peri-implantitis are induced by biofilm. Although no single candidate bacterium is responsible for the infection of any implant system ( Table 2 ), Staphylococcus aureus has been suggested to be important for dental implant failure because its specific affinity for titanium surfaces. Recent reports have shown that S aureus is an early colonizer of implant surfaces. It is found at implant sites 3 months to 1 year after implant placement. Early implant failures are reported to be associated with S aureus because of low serum antibody titers, which suggests an impaired host response to this microorganism. A cluster of bacteria, including S aureus , has been found to be more prevalent at sites of peri-implantitis (30.2%) than at healthy implant sites (14.1%), and the absence of S aureus is suggested to indicate implant health. However, a recent study investigating the presence of opportunistic bacteria at peri-implantitis sites found Pseudomonas aeruginosa in 3 of the 31 patients examined and S aureus in only one. Therefore, although S aureus and other opportunistic bacteria appear to play a role in select cases of peri-implantitis and implant failure, additional investigations are required because of the limited number of studies addressing this problem and the differences in the methods used.
| Authors | Implants (Patients) | Method | S | PDx | Clinical Measures | Results |
|---|---|---|---|---|---|---|
| Albertini et al, 2014 | 48 PI 33 T (n = 33) |
PCR Culture |
Yes | Yes | BOP/SUP Radiograph |
Pg, Pi, Tf, Td prevalence was similar at Peri-I and T sites. Pa (12%), Ca (3%), and Sa (3%) were detected Peri-I group. |
| Persson & Renvert, 2013 | 166 PI 47 HI (n = 213) |
DNA-H | Yes | Yes | PPD Radiograph |
A cluster of bacteria including Tf, Pg, Ts, Sa, Si, and Hi were higher at Peri-I sites compared with HI (30.4% vs 14.1%). History of PDx, age, and Tf were found associated with Peri-I. |
| Heuer et al, 2012 | 9 G 9 pm (n = 9) |
DNA-H | NR | No | PD BOP PIaque |
Microbial diversity of G sites was more complex than PM sites. Fusobacterium, Prevotella, Porphyromonas, Streptococcus, Campylobacter, and Neisseria were most prevalent at PM sites. |
| Dabdoub et al, 2013 | 33 HT/HI 23 HT/DI 8 DT/HI 17 DT/DT (n = 81) |
16S RNA Pyroseq. |
NR | Yes | PD BOP/SUP GI Mobility Radiograph |
Periodontal pathogens are found in 37% of the DI sites. Staphylococcus and Treponema are associated with DI compared with T sites. Most abundant species remain different between I and T sites. Geographic proximity is not sufficient to explain peri-implant microbial composition. |
| Koyanagi et al, 2013 | 6 PI 6 PDx (n = 6) |
16S RNA | No | Yes | PD BOP/SUP Radiograph |
Microbial composition of Peri-I sites was more diverse than PDx. Periodontopathogens were detected in lower amounts in Peri-I sites. |
| Cortelli et al, 2013 | 53 HI/53 HT 50 pm /50 G 50 PI/50 PDx (n = 306) |
16S RNA | No | Yes | PD CAL BOP/SUP Radiograph |
Peri-implant mucositis and Peri-I shared similar microbial composition. Composition of bacterial species was different between PI and PDx. Bacterial frequency was higher in teeth compared with implants Pg was associated with Peri-I. |
| Kumar et al, 2012 | 10 PDx 10 PI 10 HI 10 HT (n = 40) |
16S RNA Pyroseq |
NR | Yes | PD CAL BOP PIaque |
Peri-I and HI biofilms are less diverse than PDx and HT-related biofilms. PI had higher levels of Actinomyces , Peptococcus, Mycoplasma, Eubacterium, Campylobacter, Butyrivibrio, S mutans, and Treponema compared with HI and HT. Peri-I is a microbiologically heterogenous gram-negative infection. |
| Renvert et al, 2007 | 31 PI 127 pm 55 HI (n = 231) |
DNA-H | NR | Yes | PPD BOP Radiograph |
Edentulous and dentate subjects showed similar microbial profiles. Peri-I, PM, and HI sites had similar bacterial profile regardless of implant disease status. |
| Shibli et al, 2008 | 22 PI 22 HI (n = 44) |
DNA-H | No | No | Radiograph BOP/SUP |
HI sites had similar supra/subgingival plaque composition. Peri-I sites had higher levels of red complex bacteria in supra/subgingival plaque. Peri-I sites had different bacterial composition between subra- and subgingival plaque. |
| Botero et al, 2005 | 16 PI 15 HI 23 T (n = 19) |
Culture | NR | NR | PD BOP/SUP Radiograph |
Peri-I sites showed increased levels of gram-enteric rods and Pg. A correlation was found between subgingival colonization in PI and neighboring T sites for enteric rods and Pg. |
| Hultin et al, 2002 | 45 PI 53 HI 133 T (n = 36) |
DNA-H | Yes | No | PPD GI Plaque Radiograph |
Pg, Pi, Bf, Aa, and Td were detected in HI and PI sites and around teeth. Pg, Pi, Bf, Aa, and Td were detected >10 6 in PI sites. Peri-I is a site-specific infection rather than a specific host response. |
| Leonhardt et al, 1999 | NR (n = 88) | Culture | Yes | Yes | PD BOP/SUP Radiograph |
Edentulous patients have not harbored periodontopathogens. Enterics were found commonly in Peri-I sites compared with HI sites. PI sites carried more periodontopathogens HI sites. |
| Sbordone et al, 1995 | 19 FI (n = 13) | Culture | NR | NR | PD PAL BOP/SUP Plaque GI |
Fusiform bacteria, spirochetes, and motile curved rods were isolated at failing implant sites. |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


