Fig. 3.1
Confocal laser scanning microscopy image showing VSC production (red) in the deep layers of the biofilm
Biochemical Aspects
Metabolism
Oral malodor is considered to derive primarily from the anaerobic degradation of proteinaceous materials by oral microorganisms. Proteins are readily available from saliva, exfoliated epithelial cells, food debris, blood and crevicular fluids, and possibly postnasal drip. The constituent proteins are hydrolyzed by proteolytic enzymes of bacterial origin, yielding free amino acids, which can then be further broken down. Some of the molecular by-products of this process are particularly foul-smelling.
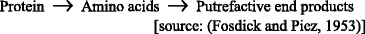
Deglycosylation: Salivary mucins are large glycoproteins comprised of a long protein core surrounded by carbohydrate side chains in a bottle brush-like structure. Unlike ordinary polypeptides, their proteolytic degradation requires the prior removal of their carbohydrate side chains, or deglycosylation
Mucin → Protein → Amino acids → Putrefactive end products
One of the key enzymes in the deglycosylation process is β-galactosidase. Our research has shown that β-galactosidase activity in saliva highly correlated with malodor ratings of 64 subjects (Sterer et al. 2002). Furthermore, addition of β-galactosidase or β-galactosidase producing bacteria (i.e., Streptococcus salivarius) to a mucin incubation mixture promoted mucin putrefaction by Porphyromonas gingivalis (Sterer and Rosenberg 2006).
The proteolytic process described above results in the breakdown of available oral proteins (or glycoproteins) into free amino acids. These free amino acids serve as nutrients for oral microorganisms that do not grow on carbohydrates (asaccharolytic), and their metabolites are important pH modulators and precursors for various molecules such as iron-scavenging siderophores. Amino acids are degraded via different metabolic pathways such as deamination, decarboxylation, and various oxidation–reduction processes, yielding different by-products, as described below.
Volatile fatty acids: The enzymatic cleavage of the amino group from various amino acids occurs at a pH range of 6–7 and results in the production of volatile fatty acids, and ammonia (NH3) (Table 3.1).
Table 3.1
Deamination products of anaerobic bacteria
|
Amino acid
|
VFA produced
|
|---|---|
|
Alanine, glycine, serine
|
Acetate
|
|
Threonine
|
Propionate
|
|
Glutamate, aspartate
|
Acetate, propionate, butyrate
|
|
Valine
|
Isobutyrate
|
|
Leucine
|
Isovalerate
|
|
Isoleucine
|
2-Methylbutyrate
|
|
Phenylalanine
|
Phenylacetate
|
|
Tyrosine
|
p-Hydroxyphenylacetate
|
|
Tryptophan
|
Indoleacetate → 3-methylindole
|
|
Tyrosine
|
Phenylacetate, phenylpropionate
|
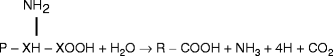
Amines: Decarboxylation of nitrogen-containing amino acids occurs in the mouth mainly at pH 6.5 (Gochman et al. 1959) and serves also as a means for the microorganisms to regulate pH conditions. The decarboxylation of these amino acids results in the production of various amine compounds (Table 3.2).
Table 3.2
Decarboxylation reactions by anaerobic bacteria
|
Amino acid
|
Amine produced
|
|---|---|
|
Glycine
|
Methylamine
|
|
Alanine
|
Ethylamine
|
|
a-Aminobutyrate
|
Propylamine
|
|
Ornithine
|
Putrescine → pyrrolidineb
|
|
Argininea
|
Putrescine → pyrrolidineb
|
|
Norvaline
|
Butylamine
|
|
Lysine
|
Cadaverine → piperidineb
|
|
Arginine
|
Agmatine
|
|
Histidine
|
Histamine
|
|
Cysteic acid
|
Taurine
|
|
Tyrosine
|
Tyramine
|
|
Tryptophan
|
Tryptamine
|
|
Phenylalanine
|
Phenylethylamine
|
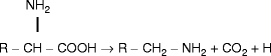
Indoles and Phenols: Production of indoles and phenols occurs as a result of the metabolism of various aromatic amino acids (Table 3.3).
Table 3.3
Indoles and phenols
|
Amino acid
|
Products
|
|---|---|
|
Tyrosine
|
Phenol, p-Cresol
|
|
Tryptophan
|
Indole, 3-Methyl Indole (Skatole)
|
|
Phenylalanine
|
Phenyl acetate, Phenyl propionate
|
Volatile sulfide compounds: Production of sulfur containing compounds results from sulfate reduction or the metabolism of sulfur containing amino acids:
Cysteine → Hydrogen sulfide
Methionine → Methyl mercaptan
Malodorous Volatile Organic Compounds (VOCs) in the Oral Cavity
Many of the above compounds, as well as various other VOCs, have been detected in saliva samples, saliva headspace, tongue-coating samples, and breath samples. These samples were analyzed using different techniques varying from classic colorimetric techniques to modern liquid and gas chromatography. These reported compounds are listed in Table 3.4, highlighting those that have been implicated in oral malodor production and stating their odor characteristics and thresholds:
Table 3.4
Oral VOCs detected in saliva, tongue coating samples, saliva headspace, and mouth air
|
Compound
|
Odor description
|
Odor threshold (ppm v/v)
|
References
|
|---|---|---|---|
|
Acids
|
|||
|
Acetic acid
Butyric acid
Methylpropionic acid
|
(2, 6)
(2, 6)
(2)
|
||
|
Alcohols
|
|||
|
Ethanol
Propanol
Decanol
Dodecanol
Tetradecanol
Hexadecanol
Phenylethanol
2-Ethylhexanol
Benzylalcohol
|
(1)
(1, 4, 5, 6)
(1)
(1)
(1)
(1)
(1)
(1)
(1)
|
||
|
Aldehydes
|
|||
|
2-heptanal
2-octanal
Nonanal
Acetaldehyde
Benzaldehyde
|
(2)
(2)
(5)
(6)
(1, 5)
|
||
|
Aromatics
|
|||
|
C2-C4 Alkyl benzenes
Ethylbenzene
Styrene
Dimethylbenzene
Benzene
Naphtalene
Toluene
|
(1)
(2)
(1, 5)
(2)
(1)
(2)
(1)
|
||
|
Ethers
|
|||
|
Dimethylfuran
|
(1)
|
||
|
Fixed gases
|
|||
|
Carbon disulfide
Dimethyl selenide
Thiopropanal-s-oxide
|
(4)
(4)
(6)
|
||
|
Hydrocarbons
|
|||
|
2-methyl-propane
dimethoxy-methane
trichloro-ethane
1(1 ethoxyethoxy)-propane
2-methyl 1-nitropropane
|
(2)
(2)
(2)
(2)
(2)
|
||
|
2,4 –pentadienenitryl
Isoprene
Caryophyllene
β-Pinene
Isobutene
Tridecane
Dodecane
Undecane
Pentadecane
Decane
Limonene
C8-C12 Alkanes
C17 Alkane
|
(2)
(5)
(5)
(5)
(5)
(5)
(5)
(5)
(5)
(5)
(2, 5)
(1)
(1)
|
||
|
Ketones
|
|||
|
2-Butanone
2-Heptanone
2-Hexanone
2-Pentanone
6-Methyl-2-heptanone
Acetone
|
(4, 5)
(2)
(2)
(4)
(2)
(1, 4, 5, 6)
|
||
|
Nitrogen containing compounds
|
|||
|
Diphenylamine
Pyrrolidine
Putrescine
|
(1)
(2)
(3, 7)
|
||
|
Cadaverine
Methenamine
Ammonia
Indole
|
Putrid
–
Pungent
Fecal
|
–
–
1.5
0.00030
|
(3, 7)
(5)
(6, 8)
(1, 2, 3, 4, 9)
|
|
2-Methyl pyridine (Picoline)
|
Sweat
|
0.031
|
(1, 2)
|
|
3-Methyl pyridine
4-Methyl pyridine
Ethyl-pyridine
|
(1)
(1)
(2)
|
||
|
3-Methyl-indole (Skatole)
|
Fecal
|
0.0000056
|
(1, 2, 3 ,4)
Pyridine
Rancid
0.063
(1)
|
|
Phenols
p-Cresol
Dimethylphenol
Phenol
|
(1, 2)
(2)
(1, 2, 5)
|
||
|
Sulfur-containing compounds
|
|||
|
2-Methyl-thio-propane
Mercapto-acetic acid (2)
Methylsulphide (2)
Propane-thiol (2)
Methylthiopropane (2)
Thiocyanic acid (2)
Ethanethioic acid-S-ME (2)
Methylbenzotiophene (2)
Benzenecarbothiotic acid (2)
Methanesulfonylazide (2)
|
(2)
(2)
(2)
(2)
(2)
(2)
(2)
(2)
(2)
(2)
|
||
|
Dimethyl sulfide
|
Cabbage
|
0.0030
|
(4, 5)
|
|
Allyl methyl sulfide
Diallyl disulphide
|
(4)
(6)
|
||
|
Hydrogen sulfide
Methyl mercaptan
Dimethyldisulfide
Dimethyltrisulfide
|
Rotten egg
Sewer
Sulfur
–
|
0.00041
0.000070
0.0022
–
|
(4)
(2, 4)
(1, 2, 4, 5)
(1, 2, 4, 5)
|
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


