Local pain management is the most critical aspect of patient care in dentistry. The improvements in agents and techniques for local anesthesia are probably the most significant advances that have occurred in dental science. This article provides an update on the most recently introduced local anesthetic agents along with new technologies used to deliver local anesthetics. Safety devices are also discussed, along with an innovative method for reducing the annoying numbness of the lip and tongue following local anesthesia.
Local pain management is, without doubt, the most critical aspect of patient care in dentistry. The improvements in agents and techniques for local anesthesia are probably the most significant advances that have occurred in dental science, enabling the profession to make tremendous therapeutic advances that would otherwise not have been possible. Today’s anesthetics are safe, effective, and can be administered with negligible soft tissue irritation and minimal concerns for allergic reactions. This article provides an update on the most recently introduced local anesthetic agents as well as new technologies used to deliver local anesthetics.
Articaine
Articaine is the newest addition to the local anesthetic arsenal, and was approved by the Food and Drug Administration (FDA) in April 2000. It is a member of the amino amide class of local anesthetics, and is the most widely used local anesthetic agent in dentistry in several European countries and in Canada. Articaine hydrochloride (HCl) is available in 4% strength with 1:100,000 or 1:200,000 epinephrine.
The amide structure of articaine is, in general, similar to that of other local anesthetics. It is unique, however, among the amide local anesthetics in that it does not contain a benzene ring like the others but instead contains a thiophene ring. The thiophene ring increases its liposolubility, making it more effective in crossing lipid barriers. It also contains an additional ester group, which enables articaine to undergo biotransformation in the plasma (hydrolysis by plasma esterase) as well as in the liver (by hepatic microsomal enzymes).
Articaine HCl does not possess any relevant systemic side effects or gross toxicity, and can be considered a safe local anesthetic. The safety and efficacy of articaine has been studied, and it has been found to be a well-tolerated, safe, and effective local anesthetic for use in clinical dentistry that will meet the clinical requirements for pain control of most dental procedures in most patients. Its lower systemic toxicity and wide therapeutic range permits the use of articaine in higher concentrations than other amide-type local anesthetics.
Articaine reaches its peak blood concentration in about 25 minutes following a single-dose dental injection by the submucosal route of a solution containing 1:200,000 epinephrine. It diffuses better through soft tissue and bone than other local anesthetics. The concentration of articaine in the alveolus of a tooth in the upper jaw after extraction was about 100 times higher than that in systemic circulation. Approximately 60% to 80% of articaine HCl is bound to human serum albumin and γ-globulins, and is rapidly metabolized by plasma carboxyesterase to its primary metabolite—articainic acid—which is secreted by the kidneys as an inactive metabolite. The elimination half-life is 20 minutes. The onset of anesthesia is within 1 to 9 minutes after injection, and complete anesthesia lasts approximately1 hour for infiltrations and up to approximately 2 hours for nerve block.
Berlin and colleagues compared the efficiency of articaine to lidocaine in a primary intraligamentary injection administered with a computer-controlled local anesthetic delivery system, and found that the efficacy of 4% articaine with 1:100,000 epinephrine was similar to the efficacy of 2% lidocaine with 1:100,000 epinephrine for intraligamentary injections. No statistically significant differences were seen in the onset and duration of anesthesia between articaine and lidocaine solutions when they were applied for maxillary infiltration anesthesia. However, extraction of maxillary teeth without palatal injection was found to be possible by depositing 2 mL articaine HCl to the buccal vestibule of the tooth rather than lidocaine, possibly due to articaine’s better bone and soft tissue penetration and diffusion. Because of its bone-penetrating ability, articaine has become popular for producing profound anesthesia in lower premolars and lower anterior teeth using localized field blocks (infiltrations) without resorting to mandibular blocks. A prospective randomized, double-blind cross-over study looked at articaine and lidocaine mandibular buccal infiltration anesthesia, and found that mandibular buccal infiltration is more effective with 4% articaine with epinephrine than with 2% lidocaine with epinephrine in achieving pulpal anesthesia. Both injections were associated with mild discomfort. Other studies compared the efficacy of articaine and lidocaine for inferior alveolar nerve blocks, showing that 4% articaine with 1:100,000 epinephrine was similar to 2% lidocaine with 1:100,00 epinephrine in inferior alveolar nerve blocks when used for regular dental procedures with no significant difference in patients with irreversible pulpitis. When the two anesthetics were compared in pediatric dental patients, a visual analog score method indicated that articaine is an effective local anesthetic in children but that it was no more effective than lidocaine.
When articaine was compared with mepivacaine 2%, the results indicated that 4% articaine was superior to 2% mepivacaine in depth of anesthesia, mainly over the first 60 minutes.
In the light of its pharmacodynamic properties, articaine can be arranged with local anesthetics similar to lidocaine.
Articaine in Pediatric Patients
The use of articaine in pediatric patients younger than 4 years is not recommended. The quantity to be injected should be determined by the age and weight of the child and the magnitude of the operation. For children younger than 10 years who have a normal lean body mass and normal body development, the maximum dose may be determined by the application of any of the standard pediatric drug formulas. In any case, the maximum dose of 4% articaine HCl should never exceed the equivalent of 7 mg/kg (0.175 mL/kg) or 3.2 mg/lb (0.0795 mL/lb) of body weight.
It is important to be careful when administering articaine to a sedated child, because there is an increased risk of adverse reactions occurring and the sedation may mask the clinical signs. There is a direct link between adverse reactions and local anesthesia volumes.
Articaine and Paresthesia
Persistent paresthesia of the lips, tongue, and oral tissues have been reported with the use of articaine HCl, with slow, incomplete, or no recovery. These adverse events have been reported chiefly following inferior alveolar nerve blocks and seem to involve the lingual nerve most often. Epidemiologic studies of permanent paresthesia following a local anesthetic injection by Haas suggested that the 4% solutions used in dentistry, namely articaine and prilocaine, are more highly associated with this occurrence. Another study by Miller and Haas published in 2000 reported that the incidence of paresthesia from either prilocaine or articaine (the only two 4% drugs on the dental market) was close to 1:500,000 injections, which is relatively low. This result suggests that there may be factors involved other than the drugs themselves.
In a detailed review of paresthesia cases due to nerve blocks evaluated by the Oral and Maxillofacial Surgery Department of the University of California at San Francisco, Pogrel reported that 35% of the cases involved the use of lidocaine and 30% involved the use of articaine. Pogrel concludes that nerve blocks can cause permanent damage to the nerves, independent of the local anesthetic used, and that he did not find any disproportionate nerve involvement from articaine but that articaine is associated with this phenomenon in proportion to its usage.
A 2005 report by the Canada Review Agency (CRA) offered conflicting numbers of paresthesia incidents involving the use of articaine. (The link of articaine with paresthesia was first reported from Canada in 1995.) Like Pogrel, these investigators also concluded that the CRA found no common factor explaining articaine-linked paresthesias.
The operator must therefore look at the evidence of reported articaine-associated paresthesia cases carefully, and be guided by ethical obligations to deliver the most outstanding care possible, without denying patients access to the better care to which they are entitled and yet not expose them to unnecessary risks.
Other Considerations
The metabolism of articaine was found to be age-independent in healthy male volunteers, therefore no change of dosage of articaine in elderly patients should be necessary, but it should be used with caution in patients with heart blocks. It should also be used with caution in patients during or following the administration of potent general anesthetic agents, because cardiac arrhythmias may occur under such conditions. Systematic allergic reactions caused by articaine have been reported. Articaine is classified in Pregnancy Category C, and should be used during pregnancy only if the anticipated benefit justifies the risk to the fetus. It is not known whether the drug is excreted in human milk, and caution should be exercised if it is administered to a nursing woman.
Oraqix (lidocaine & prilocaine periodontal gel 2.5%/2.5%)
Oraqix (Dentsply Pharmaceutical, York, PA, USA) is a topical anesthetic agent introduced in 2004, and is designed primarily for use by dental hygienists. The FDA approved Oraqix for periodontal applications. It is a needle-free subgingival anesthetic for use in adults requiring localized anesthesia in periodontal pockets during scaling and/or root-planing procedures.
Oraqix is an oil at room temperature, so it can be easily applied into periodontal pockets requiring root planing and scaling. Once applied it solidifies at body temperature into an elastic gel, enabling it to remain in place while the anesthetics take effect. It is applied on the gingival margin around the selected tooth using a blunt-tipped Oraqix applicator. Scaling and root planing may begin 30 seconds after the application, and the anesthetic effect has a duration of approximately 20 minutes.
Oraqix offers minimal risk for an allergic reaction, and it may be reapplied to a maximum of 5 treatment cartridges if longer duration of the anesthetic effect is required. Overdose reactions are similar to overdoses from injectable amides (such as lidocaine).
The Oraqix applicator cost about $36.00 ( Fig. 1 ).

Oraqix (lidocaine & prilocaine periodontal gel 2.5%/2.5%)
Oraqix (Dentsply Pharmaceutical, York, PA, USA) is a topical anesthetic agent introduced in 2004, and is designed primarily for use by dental hygienists. The FDA approved Oraqix for periodontal applications. It is a needle-free subgingival anesthetic for use in adults requiring localized anesthesia in periodontal pockets during scaling and/or root-planing procedures.
Oraqix is an oil at room temperature, so it can be easily applied into periodontal pockets requiring root planing and scaling. Once applied it solidifies at body temperature into an elastic gel, enabling it to remain in place while the anesthetics take effect. It is applied on the gingival margin around the selected tooth using a blunt-tipped Oraqix applicator. Scaling and root planing may begin 30 seconds after the application, and the anesthetic effect has a duration of approximately 20 minutes.
Oraqix offers minimal risk for an allergic reaction, and it may be reapplied to a maximum of 5 treatment cartridges if longer duration of the anesthetic effect is required. Overdose reactions are similar to overdoses from injectable amides (such as lidocaine).
The Oraqix applicator cost about $36.00 ( Fig. 1 ).

Reversing local anesthesia
In postsurgical patients prolonged soft tissue anesthesia is very desirable, and oral surgeons have combined oral nonsteroidal anti-inflammatories along with the long-acting local anesthetic bupivacaine HCl to successfully manage pain in the postsurgical period. However, many individuals who have had less invasive procedures (eg, routine restorations) have complained to their dentist at follow-up appointments of the inconvenience and annoyance of not being able to eat, drink, or talk normally for several hours after their dental visit because of their lip and/or tongue being numb. In reality, the majority of routine dental treatments are not so invasive that they require a patient to leave the dental office with residual soft tissue anesthesia that lingers for many hours. Patients having these minimally invasive procedures, as well as pediatric patients, could benefit from the reversal of the local anesthetic.
In May 2009, The FDA approved OraVerse (phentolamine mesylate) for the reversal of soft tissue anesthesia and the associated functional deficits resulting from a local dental anesthetic. OraVerse (Novalar Phrmaceuticals Inc, San Diego, CA, USA) is approved for use in both adults and children; however, it is not recommended for use in children younger than 6 years or weighing less than 15 kg (33 lbs).
OraVerse is a formulation of phentolamine, an α-adrenergic antagonist. The hypothesis for the mechanism of action is that phentolamine acts as a vasodilator, resulting in faster diffusion of the local anesthetic into the vascular system and away from the site, thereby reducing the unwanted side effects of lingering lip and tongue numbness.
Tavares and colleagues evaluated the safety and efficacy of a formulation of phentolamine mesylate (OraVerse) as a local anesthesia reversal agent for pediatric patients. These investigators found that the drug was well tolerated and safe in children 4 to 11 years old, and that it accelerated the reversal of soft tissue local anesthesia after a dental procedure in children 6 to 11 years old. The median recovery time to normal lip sensation was 60 minutes for the subjects in the study group versus 135 minutes for subjects in the control group. Tavares and colleagues noted no differences in adverse events, pain, analgesic use, or vital signs.
The phentolamine mesylate must be injected in the same volume and at the same site as the local anesthetic with vasoconstrictor is administered, for example, 1 carpule of lidocaine = 1 carpule of OraVerse. Although tachycardia and cardiac arrhythmia may occur with the parenteral use of α-adrenergic blocking agents, such events are uncommon after submucosal administration, and has not been reported with the use of OraVerse.
Delivery devices
While local anesthesia has been great for dentistry and has permitted the introduction of several sophisticated techniques, it is also one of the biggest reasons why people don’t go to the dentist: “fear of the needle.” To the public, the fear of “the needle” ranks with the fear of “a root canal.” These injections can cause pain due to the needle puncture of the oral mucosa, the pressure of the solution entering the area to be anesthetized, and the pH of the anesthetic solution. All of the technologies that have recently been introduced involve devices that try to reduce the pain of anesthetic injection, decrease the failure rates of achieving local anesthesia, and reduce the area anesthetized and thus the residual effects of the anesthesia.
Vibrotactile Devices
Pain relief by vibrotactile stimuli is a well-known phenomenon, and for many years dentists have intuitively used vibrations to decrease the pain from infiltration anesthesia in the upper jaw. The technique of pinching and shaking the cheek in an attempt to distract the brain from the discomfort of the anesthetic shot has been widely utilized with great success. Because the brain (cerebral cortex) is focused on the vibration, the perception of “pain” from the pressure of the liquid entering the tissue is decreased. The gate theory, introduced by Melzac and Wall in 1965, suggested that pain can be reduced by simultaneous activation of nerve fibers that conduct nonnoxious stimuli. Stimulation of larger-diameter fibers (eg, using appropriate pressure or vibration) can close the neural gate so that the central perception of pain is reduced. The proposal is based on the fact that small-diameter nerve fibers carry pain stimuli through a gate mechanism and that larger-diameter nerve fibers going through the same gate can inhibit the transmission of the smaller nerves carrying the pain signal. This aspect led to the theory that the pain signals can be interfered with or modified by stimulating the periphery with nonnoxious stimuli such as pressure and vibrations traveling through the larger-diameter fibers. Some of the newer local anesthetic delivery systems aimed at easing the fear of the needle take advantage of the gate control theory of pain management, which suggests that pain can be reduced by simultaneous activation of nerve fibers through the use of vibration.
Inui and colleagues have shown, however, that pain reduction due to nonnoxious touch or vibration can result from tactile-induced pain inhibition within the cerebral cortex itself and that the inhibition occurs without any contribution at the spinal level, including descending inhibitory actions on spinal neurons. Their study suggested that activation of the tactile pathway at a level higher than that in the spinal cord can inhibit cortical responses to noxious stimuli and does not rely on a gating mechanism.
There are currently 4 devices being marketed in the United States that try to reduce pain at the injection site by relying on vibrations.
VibraJect
VibraJect (Miltex Inc, York, PA) is a small device that aims to block the pain from injections through the use of vibrations ( Fig. 2 ). It is a small battery-operated attachment that snaps on to the standard dental syringe. It delivers a high-frequency vibration to the needle that is strong enough for the patient to feel. Nanitsos and colleagues found this device to be effective in decreasing injection site pain and used the gate control theory to explain their findings. Research regarding the effectiveness of VibraJect, however, has been mixed. Nanitsos and colleagues (2009), Murray and colleagues (2003), and Blair (2002) reported the device to be effective whereas Saijo and colleagues (2005) and Yoshikawa and colleagues (2003) reported no significant pain reduction when VibraJect was applied to a conventional dental syringe.
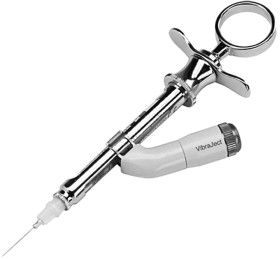
VibraJect was introduced in 2002, and is now in the general price range of US$289 to $359 (it has been seen on certain Web sites for $269). The dental retailers of this device advertise that both the VibraJect and VibraJect R3 dental syringes are equipped with rechargeable batteries with long durability. Several dentists in private practice blogs have complained about short battery life, however.
DentalVibe
DentalVibe (BING Innovations LLC, Crystal Lake, IL, USA) is a recently introduced cordless, handheld injection system, which also uses vibration diversion based on the pain gate theory ( Fig. 3 ). The device delivers a pulsed, percussive vibration with enhanced amplitude, which gently taps the mucosa in a synchronized, changing pattern. The changing pattern is supposed to keep the A-β nerve fibers activated. The DentalVibe consists of a U-shaped vibrating tip attached to a microprocessor-controlled VibraPulse motor. The activated U-shaped vibrating tip is first applied to the injection site, and the dental needle may be inserted anywhere in the vibrational zone. It also lights the injection area and has an attachment to retract the lip or cheek.
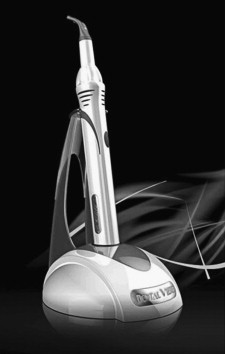
The DentalVibe sells for approximately $795.00.
Accupal
The Accupal is a cordless device made specifically for palatal injections. It uses both vibration and pressure to precondition the palatal mucosa ( Fig. 4 ). Accupal (Accupal, Hot Springs, AR, USA) provides pressure and vibrates the injection site 360° proximal to the needle penetration, which shuts the “pain gate,” according to the manufacturer. After placing the device at the injection site and applying moderate pressure, the unit lights up the area and begins to vibrate. The needle is placed through a hole in the head of the disposable tip, which is attached to the motor. It uses one AAA standard battery.
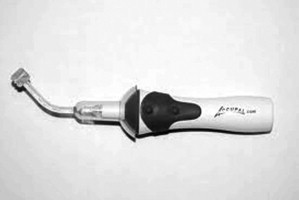
The company has recently expanded its product line with the I/A model, designed for inferior alveolar nerve blocks. The device is the same except for a special tip designed for mandibular injections. According to the company it improves success in inferior alveolar blocks. Accupal devices retails for $499.00 but have been seen online for $399.00. Tips cost about $2.00 apiece.
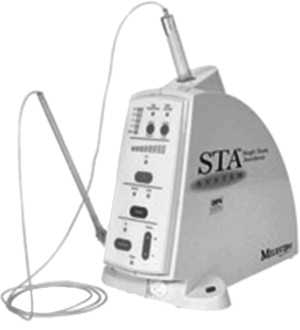
Single Tooth Anesthesia/CompuDent (WAND)
The STA (Single Tooth Anesthesia) system (Milstone Scientific Inc, Livingston, NJ, USA), introduced in 2007, also uses vibrational technology to decrease pain ( Fig. 5 ). In addition, it “tells” the dentist if the needle has been positioned properly via real-time visual and audible feedback, providing information on the exit pressure of the anesthetic and the type of tissue encountered. It also uses a computer program to control the anesthesia flow to provide low-pressure injections resulting in pain-free, precise anesthetic delivery, regardless of required pressures (loose connective vs firm connective palatal mucous membrane). The “hardware” looks similar to a miniature computer tower connected to a cartridge containing the local anesthetic. A tube connects this to a pen-like handpiece with a very tiny needle. The cartridge holder, tube, and “wand” handpiece are all single-use disposables. The unit is designed to do intraligamentary periodontal ligament (PDL) injections, replacing the dreaded needle that is used for inferior alveolar blocks with a smaller one for single-tooth anesthesia. The PDL injection allows the dentist to start working immediately after the injection is administered, resulting in uninterrupted treatment. The STA sells for about $1995.00.