Key points
- •
An infusion pump enables delivery of a smooth anesthetic consisting of propofol or propofol combined with other rapid-onset/offset agents such as ketamine and remifentanil.
- •
Maintenance intravenous therapy during anesthesia for oral and maxillofacial surgery (OMS) should consist of an isotonic crystalloid solution such as normal saline or lactated Ringer 15 mL/Kg and intravenous access with an angiocatheter as opposed to a metal needle.
- •
For patients at risk for postoperative nausea and vomiting, a multimodal approach should be used with agents that are antagonists at 5-HT 3, dopamine 2, and muscarinic receptors augmented by dexamethasone and propofol as an anesthetic.
- •
Obese patients with obstructive sleep apnea and gastroesophageal reflux disease represent an airway risk for which airway adjuncts such as a laryngeal mask airway, nasopharyngeal catheter, or tongue suture should be considered.
- •
Capnography and pretracheal auscultation with a Bluetooth pretracheal stethoscope provide essential monitoring of ventilation during office-based anesthesia.
Introduction
It is fitting that the first decade of a new millennium would witness significant advancements in office-based anesthesia in oral and maxillofacial surgery (OMS). These advancements are numerous and far reaching, and include the agents most commonly used and their method of delivery, as well as perioperative management and monitoring. In this article, some of the more significant of these advancements that have taken place during the last decade are explored. A useful tool in monitoring the changes has been the benchmark studies conducted by the American Association of Oral and Maxillofacial Surgeons (AAOMS). The first of these studies was fielded at the beginning of the last decade and the current one at the beginning of the second, in 2011 to 2012. The first study relied on volunteers and took place over several years, with a cohort of nearly 25,000 patients. Participants in the second study were chosen by a random sampling. The preliminary data from this latter study are just becoming available and currently consist of a cohort of approximately 2600 patients. Because the data from the current registry are preliminary and there are differences in study design, only general comparisons can be made to monitor trends ( Table 1 ).
| Data from 2000 a | Data from 2011–2012 b | % Change | P Value | |
|---|---|---|---|---|
| Number of Patients | 24,737 | 2577 | ||
| Procedure Performed | ||||
| Third molar | 16,892 (68.3%) | 1834 (71.2%) | ↑ 4.2% | <.003 |
| Other dentoalveolar | 17,652 (30.9%) | 566 (22.0%) | ↓ 53.1% | <.0001 |
| Implant | 650 (2.6%) | 101 (3.9%) | ↑ 50.0% | <.0001 |
| Primary Manager of Anesthesia | ||||
| Operating surgeon | 23,576 (95.5%) | 2410 (93.5%) | ↓ 2.1% | <.0001 |
| Certified registered nurse anesthetist | 654 (2.6%) | 88 (3.4%) | ↑ 30.8% | <.03 |
| Anesthesia Time (min) | ||||
| 10–30 | 14,622 (59.1%) | 1216 (47.2%) | ↓ 20.1% | <.0001 |
| 31–60 | 7588 (30.7%) | 1042 (40.4%) | ↑ 31.6% | <.0001 |
| IV Access Device | ||||
| Straight needle or butterfly | 12,218 (49.4%) | 430 (16.6%) | ↓ 66.4% | <.0001 |
| Angiocatheter | 12,313 (49.8%) | 2124 (82.4%) | ↑ 65.5% | <.0001 |
| IV Fluids | ||||
| None used | 9208 (37.2) | 0 | ↓ 100% | <.0001 |
| IV fluids used | 15,529 (62.8) | 2555 (99.2) | ↑ 50.0% | <.000 |
Comparison of the data from the 2 AAOMS studies (see Table 1 ) shows that third molar removal continues to be the most frequently performed procedure (approximately 70%). On the other hand, over the decade, other dentoalveolar procedures have decreased by more than 50% ( P <.001), whereas implant procedures have increased 50% ( P <.001). The operating surgeon continues to be the primary manager of anesthesia, and nearly 90% of the procedures performed are less than an hour in length. However, the 2011 to 2012 study suggests that there is an increase of more than 30% in procedures greater than 30 minutes in length ( P <.001), which may in part be a reflection of the increase in implant procedures.
Introduction
It is fitting that the first decade of a new millennium would witness significant advancements in office-based anesthesia in oral and maxillofacial surgery (OMS). These advancements are numerous and far reaching, and include the agents most commonly used and their method of delivery, as well as perioperative management and monitoring. In this article, some of the more significant of these advancements that have taken place during the last decade are explored. A useful tool in monitoring the changes has been the benchmark studies conducted by the American Association of Oral and Maxillofacial Surgeons (AAOMS). The first of these studies was fielded at the beginning of the last decade and the current one at the beginning of the second, in 2011 to 2012. The first study relied on volunteers and took place over several years, with a cohort of nearly 25,000 patients. Participants in the second study were chosen by a random sampling. The preliminary data from this latter study are just becoming available and currently consist of a cohort of approximately 2600 patients. Because the data from the current registry are preliminary and there are differences in study design, only general comparisons can be made to monitor trends ( Table 1 ).
| Data from 2000 a | Data from 2011–2012 b | % Change | P Value | |
|---|---|---|---|---|
| Number of Patients | 24,737 | 2577 | ||
| Procedure Performed | ||||
| Third molar | 16,892 (68.3%) | 1834 (71.2%) | ↑ 4.2% | <.003 |
| Other dentoalveolar | 17,652 (30.9%) | 566 (22.0%) | ↓ 53.1% | <.0001 |
| Implant | 650 (2.6%) | 101 (3.9%) | ↑ 50.0% | <.0001 |
| Primary Manager of Anesthesia | ||||
| Operating surgeon | 23,576 (95.5%) | 2410 (93.5%) | ↓ 2.1% | <.0001 |
| Certified registered nurse anesthetist | 654 (2.6%) | 88 (3.4%) | ↑ 30.8% | <.03 |
| Anesthesia Time (min) | ||||
| 10–30 | 14,622 (59.1%) | 1216 (47.2%) | ↓ 20.1% | <.0001 |
| 31–60 | 7588 (30.7%) | 1042 (40.4%) | ↑ 31.6% | <.0001 |
| IV Access Device | ||||
| Straight needle or butterfly | 12,218 (49.4%) | 430 (16.6%) | ↓ 66.4% | <.0001 |
| Angiocatheter | 12,313 (49.8%) | 2124 (82.4%) | ↑ 65.5% | <.0001 |
| IV Fluids | ||||
| None used | 9208 (37.2) | 0 | ↓ 100% | <.0001 |
| IV fluids used | 15,529 (62.8) | 2555 (99.2) | ↑ 50.0% | <.000 |
Comparison of the data from the 2 AAOMS studies (see Table 1 ) shows that third molar removal continues to be the most frequently performed procedure (approximately 70%). On the other hand, over the decade, other dentoalveolar procedures have decreased by more than 50% ( P <.001), whereas implant procedures have increased 50% ( P <.001). The operating surgeon continues to be the primary manager of anesthesia, and nearly 90% of the procedures performed are less than an hour in length. However, the 2011 to 2012 study suggests that there is an increase of more than 30% in procedures greater than 30 minutes in length ( P <.001), which may in part be a reflection of the increase in implant procedures.
Agents
Primary Intravenous Anesthetic Agents
The modern era of office-based anesthesia in our specialty began in the 1950s, when Hubbell and Krogh popularized the use of intravenous (IV) Pentothal (thiopental) anesthesia. Pentothal was replaced by the shorter-acting barbiturate methohexital, which continued to be the primary agent used for office-based anesthesia until the end of the century. However, when problems developed at the production facility for methohexital in the early years of the twenty-first century, many surgeons were forced to turn to propofol, which had largely replaced the barbiturates in medical anesthesia during the previous decade. Although methohexital returned to the marketplace, many of those oral and maxillofacial surgeons who had begun using propofol no longer wished to return to methohexital. Data from the AAOMS benchmarking studies ( Table 2 ) indicate that most (approximately 70%; P <.002) patients receive propofol as their primary anesthetic agent. However, at the time of the first study a decade ago, a virtually identical large majority (approximately 70%; P <.001) were receiving methohexital and only approximately 20% were receiving propofol.
| Data from 2000 a | Data from 2011–2012 b | % Change | P Value | |
|---|---|---|---|---|
| Primary Parental Drug Used | ||||
| Ketamine HCI | 5284 (21.4%) | 1187 (46.1%) | ↑ 115.4% | <.0001 |
| Methohexital | 17,086 (69.1%) | 285 (11.1%) | ↓ 83.9% | <.0001 |
| Propofol | 4768 (19.3%) | 1774 (68.8%) | ↑ 256.5% | <.0001 |
| Opioids Used | ||||
| Fentanyl | 16,142 (65.3%) | 1884 (73.1%) | ↑ 11.9% | <.0001 |
| Meperidine | 3745 (15.1%) | 77 (3.0%) | ↓ 80.1% | <.0001 |
| Remifentanil | 0 | 156 (6.1%) | <.0001 | |
| Benzodiazepines Used | ||||
| Diazepam | 5288 (21.4%) | 62 (2.4%) | ↓ 88.8% | <.0001 |
| Midazolam | 16,456 (66.5%) | 2446 (94.9%) | ↑ 42.7% | <.0001 |
| Other Medications Used | ||||
| Dexamethasone | 14,002 (56.6%) | 1679 (65.2%) | ↑ 15.2% | <.0001 |
| Glycopyrrolate | 5829 (23.6%) | 720 (27.9%) | ↑ 18.2% | <.0001 |
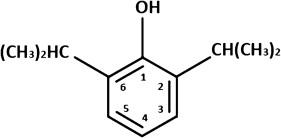
Two of the primary characteristics that have made propofol so popular with anesthesiologists and oral and maxillofacial surgeons are its rapid onset and offset. The rapid onset is largely caused by its chemical structure ( Fig. 1 ). First, the molecule is small, which allows easy passage through the blood-brain barrier ( Fig. 2 ). The second characteristic is its high lipoid solubility and its resistance to its ionization. As a so-called hindered phenol, the hydroxyl radical of propofol at carbon 1 is protected from ionization by the bulky isopropyl groups at carbons 2 and 6 (see Fig. 1 ). The offset of propofol is caused by both rapid redistribution and rapid metabolism. Historically, the parameter of elimination half-life (T ½β ) has not adequately accounted for both the rapid redistribution and biotransformation, which are in turn responsible for the rapid dissipation of the effects of such drugs as propofol. Consequently, a new parameter, the context-sensitive half-time, was developed and is described later.
Because its chemical structure provides propofol with rapid access to its receptor sites in the central nervous system (CNS), it can quickly bind to them and have a commensurate rapid onset. However, the bond is short-lived, and soon the propofol molecules return to the central circulation and pass to other parts of the body in a manner similar to that of barbiturates ( Fig. 3 ). However, not all of the body tissues have the same level of vascularity. Consequently, the tissues have been artificially divided into compartments based on their extent of vascularization. These compartments are shown in the model in Fig. 4 and include a central compartment, a vessel-rich compartment, a vessel-poor compartment, and an intermediate compartment. Once the propofol molecules have passed into the various compartments, they return to the central circulation and pass through the liver, where much of the biotransformation of the drug takes place. The biotransformed drug is eliminated through the urine. In addition, propofol has been shown to have extrahepatic metabolism, and elimination is in part through the lungs.
The context-sensitive half-time was developed to take into account the complex fate of drugs such as propofol described earlier. It is based on calculations derived from the volumes of distribution of these drugs. These volumes represent the theoretical volume into which the drug is distributed, which in turn is based on the rapidity of that redistribution. The context-sensitive half-time is a computer-generated value that represents the time required for the concentration of the drug in the plasma to decrease to a level of 50% once an infusion of the drug has been discontinued. It is then a reflection of the pattern of the dissipation of drug affect. The context-sensitive half-time plot for several IV anesthetic agents is shown in Fig. 5 . In general, those drugs that have a low, relatively flat plot are those that are associated with rapid dissipation of drug effect and the likelihood of early discharge for the patient. Thus, the more desirable plot of propofol can be appreciated when it is compared with the ultra–short-acting barbiturate agents thiopental and methohexital.
Another intriguing property of propofol is the manner in which it interacts with the γ-aminobutyric acid A (GABA A ) receptor. The GABA A receptor initially described for the benzodiazepines has proved to be the receptor for several anesthetic agents, including the barbiturates, propofol, etomidate, and inhalation agents ( Fig. 6 ). The receptor is one of the most extensively studied and , has a familiar configuration, consisting of 2 α subunits, 2 β subunits, and a γ subunit. However, there are at least 3 subtypes of the β subunit, which have been designated β 1 , β 2 , and β 3 ( Fig. 7 ). Those GABA A receptors that contain the β 3 subtype are those that are the target for high doses of propofol, which induce immobilization and general anesthesia. However, when propofol interacts with GABA A receptors that contain only β 1 and β 2 subtypes, it facilitates GABA in opening of the chloride channel in a manner similar to that which has long been associated with benzodiazepine action. This dual action of propofol is important from a clinical standpoint. Because of this characteristic, propofol can be used for deep sedation and general anesthesia at high doses and yet function as a sedative agent facilitating the action of GABA at lower doses. Thus, the agent has found a place in the sedation of older or medically compromised patients as well as those patients who are undergoing lengthy procedures such as implant or bone graft surgery.
Propofol also differs from the barbiturates in several other ways. In particular, it has antiemetic properties and provides an element of postoperative euphoria. It seems that the antiemetic effects are related to 5-HT 3 antagonism in the area postrema, the location of the chemoreceptor trigger zone. Although the mechanism for postoperative euphoria of propofol has not been clearly elucidated, initial studies suggest that it may be related to increased dopamine concentrations in the nucleus accumbens, the same locus for the generation of the pleasurable effects of recreational drugs.
Propofol is an exceptional drug that has virtually revolutionized outpatient anesthesia in medicine as well as OMS. However, like all drugs, it has its shortcomings, a principal one being its insolubility in blood. In an attempt to overcome this shortcoming, medicinal chemists have developed a prodrug of propofol, which has been termed fospropofol. A phosphate monoester was added to the propofol molecule, which renders the compound water soluble. After injection of the fospropofol into the bloodstream, there is in vivo cleavage of the phosphate ester by alkaline phosphatases, resulting in the liberation of the propofol for expression of its anesthetic effects. The enzymatic cleavage is a slow process, which may take up to 15 to 20 minutes. Thus, this innovative attempt to eliminate the problems that attend the propofol emulsion has little application in OMS.
The last decade has seen the resurgence of interest in the dissociative anesthetic ketamine. Although it was initially released in the marketplace in the 1970s, its acceptance was initially dampened by its postoperative dysphoric effects. However, it was shown that much of the dysphoria could be overcome by prior administration of a benzodiazepine. In addition, it has also been found that when ketamine is administered concomitantly with propofol, the 2 drugs have many desirable complementary effects. For instance, the tendency of ketamine to increase blood pressure counteracts the hypotensive effects of propofol. Although propofol causes respiratory depression and ketamine anesthesia is associated with minimal response to hypercapnia and provides significant bronchodilation, the postoperative euphoria provided by propofol augments the effects of the benzodiazepines in overcoming the dysphoric effects of ketamine. In addition, its context-sensitive half-time plot is similar to that of propofol ( Fig. 8 ), which correlates closely with rapid recovery and readiness for discharge. Data from the AAOMS benchmark studies suggest that these desirable attributes of ketamine have led to inclusion of the drug in the approach of many surgeons to their office-based anesthetic. The current study indicates that approximately 46% of patients receive ketamine compared with only 21% in 2000 (see Table 2 ), an increase of approximately 115% ( P <.001).
Preinduction Agents
Soon after the emergence of methohexital as a primary agent for office-based anesthesia in OMS, the first IV benzodiazepine diazepam was introduced by Leo Sternbach of Roche Laboratories. It became apparent that the inclusion of a benzodiazepine as a preinduction agent for methohexital anesthesia tended to smooth the course of the anesthetic. The phenylpiperidine synthetic opioid meperidine was added to provide the synergistic effects of a benzodiazepine/opioid combination. Over the next 2 to 3 decades, the combination of methohexital, diazepam, and meperidine gained wide acceptance. However, in the closing decades of the century, both a shorter-acting benzodiazepine and a new short-acting synthetic opioid became available. The shorter-acting benzodiazepine, midazolam, also had the desirable property of being water soluble, which significantly reduced the incidence of phlebitic complications associated with diazepam. The shorter-acting synthetic opioid fentanyl, an anilidopiperidine, soon made inroads into many offices as well.
A comparison of the AAOMS studies (see Table 2 ) indicates that there has been a nearly 90% decrease ( P <.001) in the number of patients receiving diazepam over the last decade. During the same decade, the percentage of patients receiving midazolam increased from approximately 66% to 95%, an increase of more than 40% ( P <.001). That same decade has seen a decrease of approximately 80% ( P <.001) in the number of patients receiving meperidine and an increase in the patients receiving fentanyl (see Table 2 ) from approximately 65% to 73%, an increase of approximately 12% ( P <.001).
These trends suggest that meperidine is on its way out as a component of IV anesthesia in OMS. This trend in OMS reflects the attitude of the anesthetic community worldwide. The marked decline in the popularity of meperidine is based on several undesirable side effects. One of its primary metabolites, normeperidine, is only half as potent as meperidine as an analgesic but has two-thirds of its potency as a CNS excitatory agent. Furthermore, normeperidine has a long elimination half-life, which becomes particularly problematic in patients with renal disease. Its anticholinergic effects have been shown to lead to confusion as well as delirium and memory deficits, especially in the elderly. Because of these significant shortcomings, it is likely that the use of meperidine will continue to decrease.
The inclusion of benzodiazepines in a balanced IV anesthetic has had several desirable consequences. The benzodiazepines provide anxiolysis, sedation, and amnesia, which tend to make the IV anesthetic experience more comfortable for both the patient and the operator. There seem to be few downsides to their use, because they also cause little cardiorespiratory depression. However, there is growing concern that anesthesia in older patients may sometimes lead to delirium and cognitive dysfunction. Studies suggest that even short ambulatory procedures such as those that are common in OMS may be as likely a setting for these complications as general anesthesia. Benzodiazepines are viewed as a primary culprit. Because of these concerns, several prominent authorities in the field suggest that dosages of benzodiazepines should be markedly decreased or that benzodiazepines should be withheld altogether in the elderly. In the past, because of their presumed safety profile, benzodiazepines have played a prominent role in sedation for the elderly. It seems that a more reasonable alternative may be propofol in low, sedative doses.
Another concern in the use of preinduction and ancillary agents has been the respiratory depression that accompanies all opioid agents. This depression is of special concern in the sedation of both the elderly and of patients with morbid obesity and obstructive sleep apnea (OSA). Opioid-induced respiratory depression has been implicated in several fatalities. In these patient populations, markedly reduced dosages must be used and the use of nonopioid analgesics considered. An exciting innovation has been the introduction of the esterase-metabolized anilidopiperidine remifentanil ( Fig. 9 ). Because esterases are found throughout all of the tissues of the body, enzymatic transformation of remifentanil is rapid and there is no wait for prolonged biotransformation in the liver. This finding is mirrored in its low, flat plot of context-sensitive half-time ( Fig. 10 ). However, the rapid offset of remifentanil introduces consideration of the use of an infusion pump for its administration, which is discussed later.
Anesthetic Premedications
During the last decades of the last century, oral anesthetic premedications for anxious patients usually consisted of relatively long-acting benzodiazepines such as diazepam. During the first decade of the current century, diazepam was replaced in many practices by the shorter-acting benzodiazepine triazolam. After the initial release of the drug, isolated poorly substantiated reports of alleged tendency of triazolam to exacerbate suicidal tendencies caused much furor. It was shown that triazolam had the same safe track record as the other benzodiazepines. Consequently, the drug has become the favored drug for premedication in dentistry, including oral sedation for patients undergoing nonsurgical procedures by general dentists, periodontists, endodontists, and other practitioners who do not have training in general anesthesia. The medication has been used effectively as oral premedication in OMS. It comes in several strengths, including 0.125 mg, 0.25 mg, and 0.5 mg. Often, the 0.125-mg dose intended for geriatric patients is sufficient for premedications of younger patients as well.
Although benzodiazepine premedication provides sedation and anxiolysis, even triazolam with its short duration of action can prolong recovery. In addition, in patients who are particularly sensitive to the effects of benzodiazepines, their synergistic activity with opioid analgesics can exacerbate the respiratory depressant effects of the opioids. These effects have led to resurgence in interest in clonidine as a preoperative medication for OMS procedures. The medication was initially introduced as an antihypertensive agent, but many patients complained of its sedating effects. Anesthesiologists in Europe explored the possibility of using clonidine for anesthesia premedication, and found it to be efficacious. Clonidine is an α 2 agonist with sedative properties similar to natural rapid eye movement (REM) sleep, and it does not cause respiratory depression. In addition, it tends to attenuate the tendency of certain anesthetic medications, especially ketamine, to cause tachycardia and hypertension. As an α 2 agonist, it has other desirable qualities as well, including antiemetic and analgesic effects. Inclusion of ketamine in IV anesthetic techniques for OMS has increased dramatically over the last decade. Because of the various properties of clonidine discussed earlier, many surgeons have found that it provides an excellent premedication for patients who receive ketamine. In addition to providing sedation and counteracting the hypertensive and tachycardic effects of ketamine, it provides protection against the emetic effects of ketamine as well.
Medication delivery
Since its inception in the middle of the last century, IV anesthesia in OMS has been largely delivered by the so-called incremental bolus technique. Small increments of the various IV drugs are administered manually with syringes periodically during the procedure. Because of the more rapid dissipation of the effects of propofol compared with methohexital, the increments are usually smaller and more frequently administered. However, no incremental bolus technique can provide the same constant level of plasma concentration as that provided by an infusion pump. Initially, infusion pumps were cumbersome and complex, and it took several decades of technological advancements to overcome these shortcomings. However, within the last 2 decades less cumbersome and more user-friendly syringe-based infusion pumps have been developed. The most popular of these has been the Baxter Infuse O.R. syringe pump (Baxter Healthcare Corporation, Deerfield, IL, USA) ( Fig. 11 ), which has found a home in outpatient surgery centers and hospitals throughout the country. It is a hybrid digital-analog device, which delivers a constant infusion through a syringe driven by an electronically controlled advancement screw. The device proved to be simple to program and provided reliable, constant drug delivery. However, 2 shortcomings of this type of infusion system have prompted Baxter to discontinue its production of the unit. In the modern era, new demands have been placed on infusion pumps. First, it must be possible for them to interface with a computer so that information from the pump can be incorporated into the electronic medical record. Second, pump-related complications have led to recommendations that pumps have a confirmation mode. This recommendation means that once a parameter for drug delivery (eg, rate of administration) has been entered into the pump, it must be confirmed by the anesthetist. The new pumps that have entered the market place are totally digital ones, which can interface with the electronic medical record and have a confirmation code.
Regardless of whether the operator delivers their IV anesthetic by an incremental bolus technique or with a pump, the object is to provide a constant blood level of the anesthetic agent. Over the last couple of decades, technologic improvements have made it possible for this goal to be accomplished with simple, easy-to-use infusion pumps, which have become almost universally accepted in hospitals and outpatient surgery centers. However, the long, ostensibly successful, track record of the incremental bolus technique in OMS has made most oral and maxillofacial surgeons reluctant to adopt the new technology. The number of oral and maxillofacial surgeons using infusion pumps is sufficiently low that use of a pump was not included in either of the AAOMS benchmark studies. However, unofficial polls conducted at anesthesia meetings suggest that an increasing number of oral and maxillofacial surgeons are beginning to use infusion pumps. With ever-increasing interest in rapid street readiness after office-based anesthesia with such drugs as propofol and remifentanil, infusion pumps are a logical choice. Medications such as propofol and remifentanil perform best when the receptors for these drugs are constantly bathed by the circulation of the agents in the bloodstream. Lapses in the plasma concentration lead to both patient movement and patient awareness, both of which are best minimized with an infusion pump.
Although infusion pumps for total IV anesthesia have been widely embraced by anesthesiologists, the few studies reporting on their use in the OMS literature have not shown an overwhelming difference in patient response to anesthetics delivered by an incremental bolus versus an infusion pump. For an anesthetic model based on the delivery of anesthesia by the operating surgeon, a true double-blind study is virtually impossible to design. In those studies that have been performed, the difference between the 2 delivery techniques has been masked to some extent by the sedation and amnesia provided by preinduction administration of benzodiazepines and opioids. Furthermore, no studies have included balanced anesthetic techniques, in which propofol is combined with ketamine or remifentanil. Thus, further study in the application of infusion pumps for IV anesthesia in the OMS is warranted.
Modern infusion pumps incorporate considerable smart technology, which simplifies delivery of anesthetic agents. There are 2 syringe pumps that are most commonly purchased by oral and maxillofacial surgeons through equipment supply houses: the Medfusion 3500 (Smiths Medical, Dublin, OH, USA) ( Fig. 12 ) and Aitecs 12S Pro (Aitechs-USA, Kernersville, NC, USA) ( Fig. 13 ). Of the 2, the Medfusion pump has a more comprehensive drug library, and it has found a home in many hospitals and outpatient surgery centers. The Aitecs pump has a smaller drug library and fewer delivery options, but those available are more than adequate for the average OMS office. Consequently, our comments are addressed to the latter pump.
Before using an infusion pump, it is necessary for the operator to perform some simple steps, as shown in Figs. 13 and 14 . In Fig. 1 , loading of the syringe on the pump is shown. The pump is smart designed to sense the brand and size of the syringe (see Fig. 14 ). Plunger advancement is via a screw drive that is controlled by a digitally activated motor. There is a light-emitting diode (LED) window in the upper left-hand corner of the face of the pump, in which parameters are shown during the programming and operation of the pump. Nine parameters can be programmed, including the size of the syringe, the drug name (see Fig. 14 ), dosing mode, drug concentration, patient weight, infusion rate, bolus rate, bolus dose, and the occlusion level. Occlusion level represents the pressure that must build up in the line when it becomes occluded before the alarm sounds.

Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


