Technological advancement in bone grafting procedures using purified proteins or stem cells to induce osteogenesis is a significant contribution to patient care. Patients who would otherwise not have been suitable candidates for major autologous bone grafting procedures can continue to benefit from implant reconstruction, with a less debilitating bone reconstructive procedure.
Implant placement for the rehabilitation of edentulous spaces is now often viewed as the preferred treatment alternative; however, this technique requires an adequate bone site to allow for proper osseointegration.
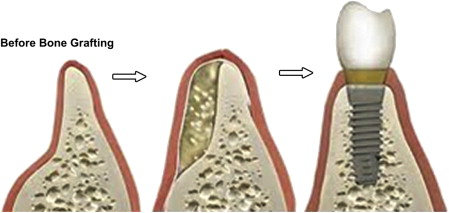
Oral and maxillofacial surgeons and general dentists have been searching for the ideal hard tissue bone graft other than autogenous bone to aid in alveolar ridge augmentation or socket preservation before implant placement for years. All sorts of alloplastic materials and allografts ( Table 1 ) have been used, but none are ideal, especially when the amount of bone needed to be added is significant or when the recipient site is significantly deficient (as in a 1- or 2-walled bony defect) ( Fig. 1 ). Autogenous bone grafts from the hip and tibia do work well, and are considered to be the gold standard for large bony defects. However, the procedure usually requires hospitalization and general anesthesia and has serious potential postoperative complications associated with the donor site, which increase in the elderly patient population, (who are the most in need of this procedure before implant placement, making them less likely to agree to undergo such a procedure) ( Box 1 ). Thus, the need for finding graft material that is osteoinductive (see Box 1 ; Table 2 ) and can help reduce the need for autogenous grafting became essential.
| Graft Material | Characteristics | Examples |
|---|---|---|
| Allograft | A graft that is taken from a member of the same species as the host but is genetically dissimilar | Cadaver cortical/ cancellous bone, FDBA, DFDBA |
| Xenograft | Graft derived from a genetically Different species than the host | Bio-Oss, coralline HA, red algae |
| Alloplast (synthetic materials) | Fabricated graft materials | Calcium sulfate, bioactive glasses, HA, NiTi |
-
Osteogenesis
-
Osteogenesis refers to living cells, such as osteoblasts, that form new bone. The success of any bone grafting procedure is dependent on having enough bone-forming or osteogenic cells in the area. Iliac Crest Bone Graft (ICBG), a type of autograft, is the only bone graft that contains enough cells to be considered osteogenic. Local bone from the primary surgical site generally contains cortical bone with much fewer cells. However, the presence of mesenchymal stem cells does not make a bone graft osteogenic. These stem cells require a signal, such as bone morphogenetic protein (BMP), to differentiate into osteoblasts.
-
Osteoconduction
-
Osteoconduction refers to the ability of some materials to serve as a scaffold onto which bone cells can attach, migrate, grow, and divide. In this way, the bone healing response is conducted through the graft site, just as a vine uses a trellis for support. Osteogenic cells generally work much better when they have a matrix or scaffold for attachment. Ceramics are strictly osteoconductive scaffolds and fall in the category of autograft extender or bone void filler.
-
Osteoinduction
-
Osteoinduction refers to the capacity of normal growth factors in the body to attract, proliferate, and differentiate primitive stem cells or immature bone cells to grow and mature, forming healthy bone tissue. Most of these signals are part of a group of protein molecules called BMPs, which are found in normal bone. Highly osteoinductive bone grafts have been evaluated as an autograft alternative in certain indications.
| Characteristic | Graft Material |
|---|---|
| Osteogenesis | Autograft |
| Osteoinduction | BMP DFDBA DBM |
| Osteoconduction | Bio-Oss Calcium phosphates Calcium sulfate Collagen FDBA Glass ionomers HA NiTi BMP |
BMPs are members of the transforming growth factor-β superfamily that were first described by Urist after observing ectopic bone formation in a rodent model from implanted devitalized cadaveric bone. This family of proteins is highly osteoinductive and contains powerful stimulants of endochondral and intramembranous bone formation from pluripotent mesenchymal cells. Several types of BMPs were isolated and cloned using recombinant DNA technology; however, the most studied has been bone morphogenic protein-2 (rhBMP-2). BMPs act as growth and differentiation factors and chemotactic agents. BMPs stimulate angiogenesis and migration, proliferation, and differentiation of mesenchymal stem cells into cartilage and hard-forming cells in an area of bone injury.
The identification and development of bone recombinant human bone morphologic protein-2 (RhBMP) has led to the commercial availability for the first time of an osteoinductive autograft replacement (Infuse Bone Graft, Medtronic Spinal and Biologics, Memphis, TN, USA). Infuse bone graft is cleared for use in interbody spine fusion, fresh tibial fractures, and oral maxillofacial bone grafting procedures.
In March 2007, Infuse bone graft was approved by the US Food and Drug Drug Administration (FDA) as an alternative to autogenous bone graft for use in sinus augmentation and localized alveolar ridge augmentation, for defects associated with extraction sockets. Prior to FDA approval, extensive preclinical and clinical research was performed to examine the feasibility, safety, and efficacy of using Rh-BMP-2/ absorbable collagen sponge (ACS) for treating common oral maxillofacial defects. These studies were first performed in several animal species, and they were followed by clinical investigation. The work demonstrated that Rh-BMP-2/ACS was effective in inducing viable de novo bone formation.
Two major clinical studies were performed to examine the feasibility, safety, and efficacy of using Rh-BMP-2/ACS. Boyne and colleagues and Fiorellini and colleagues performed randomized prospective controlled studies on the use of Rh-BMP-2/ACS in maxillary sinus augmentation procedures and in extraction site augmentation. Both studies demonstrated that Rh-BMP-2/ACS at 1.5 mg/cc induced significant bone formation suitable for implant placement. The bone induced by Rh-BMP-2/ACS was found to be biologically similar to native bone and capable of implant osseointegration and supporting the functional loading of a dental prosthesis.
Safety assessments
In the clinical study by Boyne and colleagues, those patients in the RhBMP-2/ACS treatment group did experience a significantly greater amount of facial edema than those in the bone-grafted treatment group. This edema is thought not to be the result of surgical trauma or an allergic response but rather due to the activity of the RhBMP-2 causing an influx of fluid and cells into the treatment area related to the chemotaxis and neovascularization of the site. Other than the facial edema, none of the other reported events were felt to be related to the RhBMP-2.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


