Chapter 9
Treatment of the necrotic pulp
Introduction
This chapter details the procedures utilized to carry out conservative root canal therapy (RCT) of teeth with necrotic pulps. The treatment principles described apply to teeth where the root canal system may or may not be infected. Treatment of non-infected cases is a prophylactic measure to prevent colonization and multiplication of microbes in the more or less empty root canal space and the subsequent development of symptomatic or non-symptomatic presentations of apical periodontitis (Chapter 7). In most instances, however, RCT is curative and initiated to eliminate a root canal infection, thereby remedying periapical inflammatory tissue lesions (Fig. 9.1). Treatment in such cases is also essential to impede the spread of root canal bacteria and their products to distant organs (Chapter 8). Although extraction of the tooth in question solves the infection problem, it is a radical procedure not usually acceptable to patients. RCT offers a realistic alternative, provided the tooth is restor-able. Hence, if properly conducted, RCT of teeth with infected pulp necrosis enjoys a high rate of success and can be expected to result in complete resolution of clinical and radiographic signs of apical periodontitis in almost nine out of ten cases (29, 56).
Objectives and general treatment strategies
The overall objective of RCT is to exclude the root canal system as a source of microorganisms. Therefore a primary task includes efforts to eradicate bacteria resident in the root canal system (Fig. 9.2). A second charge is to provide measures to ensure that root canal infection will not recur. The procedure includes several phases, of which mechanical instrumentation to clean and shape root canals (Chapter 11), irrigation and disinfection are critical elements. To prevent reinfection, a filling is subsequently placed in the instrumented root canal(s) (Fig. 9.1b).
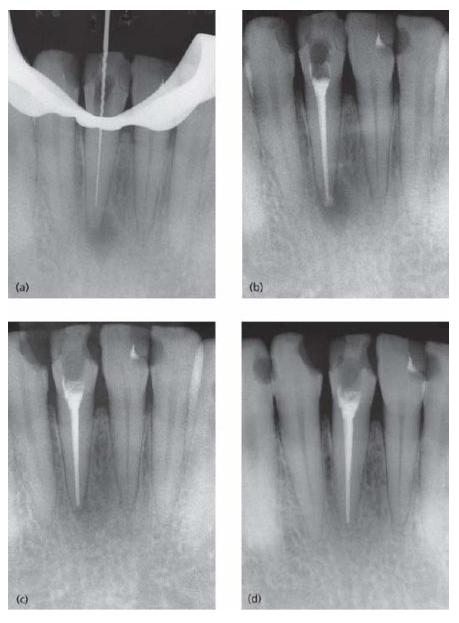
Fig. 9.1 The reason for carrying out root canal therapy of teeth with necrotic pulps is either preventive or curative. Radiograph (a) shows a second lower premolar with a well-delineated apical radiolucency in a 57-year-old woman, indicating infected pulp necrosis and apical periodontitis. The neighboring root filled first premolar also has an apical lesion suggesting an unsuccessful prior root canal treatment. Radiograph (b) is a follow-up radiograph taken 24 years after completion of endodontic treatment of both teeth that were included as abutments in a bridgework. There is now no evidence of apical bone lesions. The case demonstrates the potentials of carefully conducted RCT for a long-term successful outcome.
If successfully conducted, teeth with clinical symptoms of apical periodontitis (tenderness, pain, swelling, fistu-lae) become non-symptomatic. Successful treatment also demands that any radiographic evidence of apical peri-odontitis that existed prior to treatment should resolve and result in complete reorganization of the periapical tissue (Figs 9.1b and 9.3).
Historical perspective
Over the years, different approaches to combat end-odontic infections have been in vogue. At the beginning of the 20th century, following the discovery of the role bacteria play in disease processes in general and apical periodontitis in particular, emphasis was placed on the use of strong antiseptics. Such agents were phenol derivatives and their mixtures, e.g. methylacresylacetate and camphorated monochlorophenol, and formaldehyde and its derivatives, e.g. formocresol and paraformalde-hyde (12). Antiseptic agents were administered either to the orifice or to the interior of root canals in paste or liquid form. By evaporation of the medicament, bacterial organisms could be killed without a cleaning procedure or filling of the root canal system. In other modes of treatment strong antiseptics were combined with mechanical instrumentation, followed by filling the instrumented canal(s) with a paste containing a strong antiseptic agent. It was felt that incorporation of antiseptics in the root filling material would allow a continuous release of the antiseptic agent which would prevent survival and regrowth of any organisms that had not been killed by the instrumentation procedure. Indeed, such treatment approaches gained great popularity and are practiced even today. However, the use of strong antiseptics in endodontics raises several serious concerns:
Fig. 9.2 (a) An extracted tooth with attached inflammatory soft-tissue lesion. On cracking the tooth open (b) and observing the interior (c, d) in the scanning electron microscope, various forms of bacterial morphotypes may be identified on the root canal walls, including filaments, spirochetes, rods and cocci. (Figures (b)–(d) are from Molven et al. (37) and published with permission of Munksgaard.) (Courtesy of Dr O. Molven.)
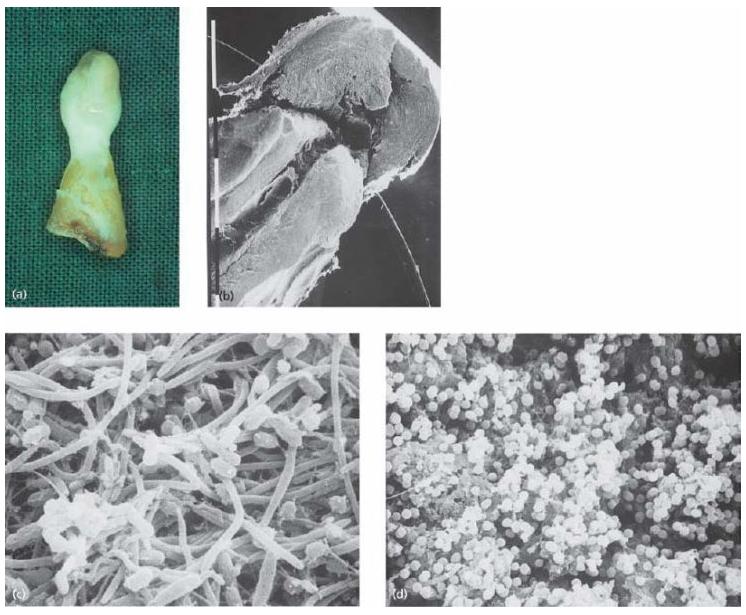
Fig. 9.3 A series of radiographs demonstrating the successful outcome of root canal therapy in a lower incisor. (a) A root canal instrument is placed in the canal, which is used to clean the canal and determine the length of instrumentation in relation to the radiographic apex. In this case working length had to be extended. Note the apical radiolucency, indicating apical periodontitis. (b) The instrumented canal has received a slightly overextended root filling. (c) A radiograph taken 2 years later. (d) Radiograph taken 6 years later, showing complete resolution of the previous lesion. The tooth now rests on a healthy periodontium and there are no symptoms (tenderness, pain, swellings) suggesting ongoing root canal infection.
- Although they are effective against microbes, strong antiseptics cause substantial cell and tissue damage, particularly if extruded to the periapical tissue environment (59) (see also Chapter 12).
- These disinfectants, also in diluted form, have the disadvantage of sensibilization and hypersensitivity responses (Chapter 12). Some chemicals even carry carcinogenic and mutagenic risks (32).
- In liquid form, chemicals are rapidly inactivated by inflammatory exudate and therefore provide antibacterial effects only of a short duration, becoming inactive within hours or a few days (22, 34).
- Since antiseptics included in root filling materials will eventually lose their antibacterial activity infection can reappear if canals were improperly filled or poorly sealed coronally, or both.
In recent decades the strategy for treatment of necrotic and infected pulps has changed in the move to more biocompatible methods. Thorough biomechanical instrumentation, combined with extensive irrigation with light antiseptics and with the use of minimally toxic and allergenic disinfectants, is now emphasized and will be detailed here.
Scheme for a routine procedure in root canal therapy
To achieve an optimal result by RCT, several critical steps can be identified:
Access opening
Once a decision for treatment has been taken and the difficulties assessed as to the presence of canal obstacles, length of canals and extent of curvatures, the root canal system needs be accessed. The most important quality of the access opening preparation is to uncover all the canal orifices present, so that an unobstructed mechanical preparation of each root canal can be carried out (see further Chapter 11). It is often advantageous to enter the tooth interior prior to the placement of a rubber dam to reduce the risk of going in the wrong direction and causing a perforation to the periodontal ligament space. Aligning the direction of the bur to the long axis of the tooth facilitates the procedure. This is particularly significant in the teeth of elderly patients, where the pulp chamber is often reduced by mineralization and is then difficult to find. It applies also to teeth with artificial crowns.
Aseptic technique
Even though teeth with necrotic pulps are most often infected, RCT requires an aseptic tech nique of opera tion. Asepsis is maintained first of all to exclude con tamination with organisms that have greater resistance to treatment than members of the root canal micro-biota. Common contaminants, which are difficult to manage, belong to the facultative Gram-positive segment, most notably enterococci; other enteric bacteria and yeasts may be introduced into the canal system owing to failure to maintain proper asepsis. Other sources of contaminating organisms are along leaky temporary restorations applied between treatment sessions and by leaving canals open to the oral cavity for drainage (54, 65). Elimination of microorganisms from root canals naturally requires prevention of oral contaminants. As stated in Chapter 4, procedures in this context include removal of plaque and calculus, defective fillings and crowns and carious dentin prior to the initiation of treatment. For the subsequent RCT, proper rubber dam application is indispensable and sterile burs and instruments must be used.
Mechanical instrumentation
Cleaning the canal interior with hand and rotary instruments is a most important technique to remove the bulk of the infecting bacterial mass and its nutritional supply. The instrumentation, if possible, should be carried out throughout the entire extension of each root canal and ideally end at its exit or slightly short of the apical foramen. The procedure aims to:
- Physically remove as much as possible of the bacterial mass, including those bacteria attached to the root canal walls in a biofilm structure and those free-floating in the canal.
- Remove sources of substrate for bacterial regrowth and multiplication, including necrotic tissue and tissue-breakdown products.
- Remove the inner portion of the root canal walls, where dentin is most heavily infected.
- Provide access for irrigation solutions to all parts of the root canal system for cleaning and chemical disinfection.
- Create a clean and properly shaped canal that facilitates the insertion of a well-sealing root filling.
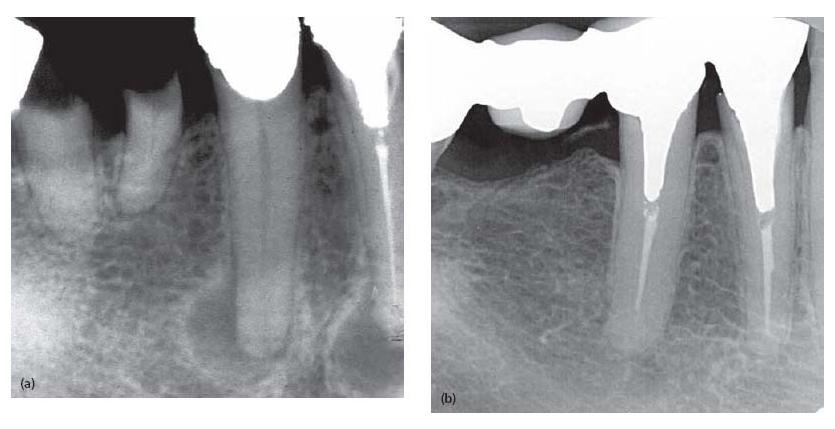
Fig. 9.4 Cross-sectional cut through a root canal partly filled with guttapercha. Note the unfilled lateral extensions (arrow heads), which may provide space for bacterial growth and leakage of bacterial elements to the periapical tissue environment.

The task is not an easy one. Not only are micro organisms located in the main canal(s) (Fig. 9.2) but they will also enter any space and ramification available to them, including dentinal tubules (45), isthmuses and lateral canals. This makes the cleaning and disinfection procedure precarious as well as demanding. In this context it needs to be recognized that crevices and lateral areas of oval-shaped canals (Fig. 9.4) are especially difficult to reach. If untouched by the instruments, both substrate and bacterial organisms may remain in such locations and, if they are allowed a pathway to the apical environment, a failure may ensue. In fact, studies examining the extent to which root canals are rendered clean after instrumentation often find remnants of necrotic tissue and debris on the canal walls, especially in oval-shaped canals (69) and in isthmuses of mesial roots of mandibular molars (39). Bacteria lodged in dentinal tubules also may remain unaffected.
Narrow, partially blocked canals and canals in severely curved roots further challenge the instrumentation procedure (Fig. 9.5a, b). Instrumentation is also a demanding task where canals are extremely wide, e.g. in young immature teeth (Fig. 9.5c). One reason for this is that the armamentarium normally is not designed for treatment of teeth with incomplete root development. Another is that instrumentation in such cases has to be limited owing to the already thin root structure to limit risk for subsequent root fracture. Therefore, combating infection in such root canals has to rely more on chemical disinfection and proper root filling than the mechanical instrumentation per se (see also Chapter 11).
The instrumentation procedure is a highly important step in RCT. By enlarging and preparing canals and giving them shape for access to irrigants, chemical disinfectants and filling, the bulk of the infecting microbiota is physically removed (3-6). Therefore the time spent in cleaning and shaping root canals according to the principles described in Chapter 11 is well worthwhile.
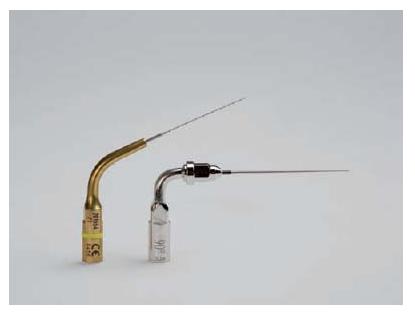
To enhance the cleaning capacity over and beyond what the mechanical preparation with hand and rotary instruments can do, it has been shown that ultrasonic activation of the irrigating solution has a positive effect. It is important that the ultrasonic instrument moves freely within the canal without active preparation, so-called passive ultrasonic irrigation (64). This measure is therefore applied after the mechanical instrumentation. The use of a small ultrasonic file, preferably with a smooth surface in order to prevent damage to the canal wall or ledge formation, is advocated (Fig. 9.6).
Considerations in routine cases
The instrumentation technique in routine cases, i.e. when the canal anatomy is within a fairly normal range in terms of width, length and curvature (Figs 9.1 and 9.3), is no different to that carried out in conjunction with pulpectomy (Chapter 4). Yet, there are certain precautions that need be undertaken to avoid three complications:
- Blocking the canal patency.
- Causing an endodontic flare-up.
- Overextending the apical foramen.
Blocking the canal patency can occur by fracturing an instrument, by causing a ledge or pushing dentin debris into the apical portion of the canal. These complications are particularly common in narrow and curved canals and are often the result of improper technique. Obviously, effective removal of the infecting microbiota is hampered by such errors. Therefore it is important that the instrumentation procedure follows a well-proven scheme of steps (see Chapter 11).
To reduce the risk of causing an endodontic flare-up (Chapter 7) and overextension of the apical foramen, proper determination of the length of instrumentation (working length) is especially important in RCT (Core concept 9.1). This measure is undertaken primarily to ensure that the entire length of each canal is treated, if possible. If instrumentation is carried out too short, substantial amounts of bacteria may be left behind and continue to sustain apical periodontitis. Indeed, this is a major cause of failure in RCT (56).
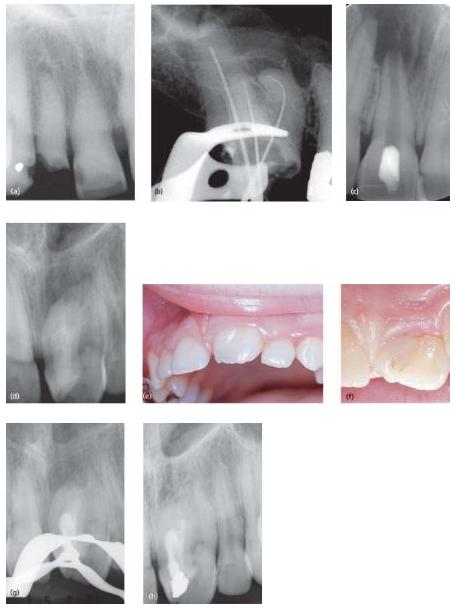
Fig. 9.5 Examples of cases displaying various degrees of difficulty: (a) a partially blocked canal in 12 due to previous mineralization processes in the pulp; (b) a tooth with severely curved root canal anatomy in the mesial root; (c) a tooth with incomplete root development; (d) a supernumerary tooth with a dense invaginatus fused to the permanent incisor; (e) buccal aspect; (f) lingual aspect. Following instrumentation and filling of the invagination only (g), the periapical lesion resolved (h). The tooth responded to an electronic pulp tester and cold, indicating that the other root portion had a vital pulp.
Fig. 9.6 Examples of small instruments for ultrasonic activation of root canal irrigant.
- Bacterial organisms and infected debris may be extruded into the periapical tissue and cause a flare-up of a non-painful lesion, aggravate a painful lesion and/or perpetuate apical periodontitis on a long-term basis.
- It may result in enhanced nutritional supply of any remaining organisms and boost their growth to cause endodontic flare-ups and/or long-term failure.
- It enhances the risk of overfilling.
- The potential to carry out a permanent root filling, which seals the apical portion bacteria-tight, is often impaired.
Careful working length determination is also important to prevent a set of complications that may ensue if instrumentation is carried out beyond the apical foramen (Core concept 9.2). One complication relates to the risk of extruding bacteria and infected dentin debris into the periapical tissue. If especially virulent, such organisms may aggravate a periapical inflammatory condition and cause the development of painful symptoms, including an apical abscess (endodontic flare-up). Extruded infected debris may also perpetuate apical periodontitis, despite complete elimination of bacterial organisms from the canal system itself (70).
Over-instrumentation also extends the apical foramen and promotes entry of inflammatory exudate into the canal. Owing to its content of serum proteins, the growth of proteolytic organisms is then likely to be boosted. This latter mechanism may also lead to an endodontic flare-up.
Another grave complication of overpreparation is so-called apical zipping (Chapter 11). This is when the canal orifice has been not only enlarged but also transported in a lateral direction. Similar to an incompletely developed root, such canals are extremely difficult to fill properly. Besides the risk of overfilling, the root canal filling is often unable to provide an apical seal (68). In fact, unfilled spaces (pockets) often remain along the apical portion of the root filling, where bacteria may continue to grow and maintain apical periodontitis (40).
Controlling pain during instrumentation
Usually treatment of a necrotic pulp does not require anesthesia. However, even in the presence of a radio-lucency, functional sensory nerve fibers may remain in the apical portion of the canal (33). Once canal instruments touch these fibers, a pain response is initiated. Thus, for painless completion of treatment, anesthesia may be required. Some patients may allow the necessary instrumentation without anesthesia, but this should not be conducted unless complete agreement with the patient has been sought. Pain is usually gone after instrumentation with one or two file sizes.
There is some benefit in carrying out the treatment without anesthesia for length control. Hence, a pain response during instrumentation of a necrotic pulp may indicate that the foramen has been inadvertently pierced and that the working length should be reassessed. This should be carried out in spite of what appears to be a correctly recorded working length. Distinction between nerve fiber remnants and overpreparation is not always obvious and to ensure proper length of instrumentation one may:
In other words, because of the sometimes deviant exit of the apical foramen into the periodontium, it is advantageous to carry out RCT in the absence of anesthesia because this draws attention to the risk of overinstru-menting the canal. Yet, the comfort of the patient should always be given the highest priority.
Irrigation and chemical disinfection
Mechanical instrumentation of root canals needs to be supported by frequent irrigation. There are several important purposes of such a measure:
- To clean out debris and dentinal shavings and to keep canals moist so that instruments run smoothly.
- To exert antibacterial effects.
- To augment the efficacy of the instrumentation procedure by dissolving necrotic tissue remnants, especially in areas mechanical instrumentation cannot reach, including crevices, isthmuses and accessory canals.
- To dissolve the smear layer.
Another desirable property of the agent to be used is that it should cause minimal tissue damage and thus be minimally toxic in case it is extruded into the periapical tissue environment.
Various irrigating solutions are available, but none can be said to satisfy all these requirements. Furthermore, it should be recognized that efficacy of agents as far as their disinfecting ability is limited by interactions with dentin, dentin debris and organic compounds present in the root canals of necrotic pulps, viz. serum proteins and microbial biomasses. This means that endodontic disinfectants may not be as potent as indicated by trials carried out in vitro (25). Commonly used irrigants are sodium hypochlorite (NaOCl) and ethylenediaminetetraacetic acid (EDTA). The specific properties of these agents will be described here in some detail.
Sodium hypochlorite
The most commonly employed solution for endodontic irrigation is NaOCl, which unites three important qualities essential to RCT:
- It dissolves organic material.
- It is a potent disinfectant.
- It is minimally irritating to tissue in low concentrations.
The tissue-dissolving capacity of NaOCl is well established (2, 38). Both vital and necrotic tissue are affected and dissolved in excess of NaOCl. The speed of tissue dissolution is dependent on the extent of contact between active solution and tissue. Thus, stirring or the use of ultrasound will speed up the tissue-dissolving process considerably (38).
The effect of NaOCl is quickly inactivated in the presence of oxidizable material, such as dentin debris and organic material, because it dissociates into Na+ and Cl– ions (24, 38). Therefore, during RCT, the solution has to be replenished consistently. Although NaOCl breaks down collagen, it hardly affects the canal walls (23, 52). The addition of surfactant, which should promote its flow into areas inaccessible for mechanical instrumentation, or hydrogen peroxide has not been confirmed to provide significant therapeutic effects.
Sodium hypochlorite is a strong and fast-acting disinfectant with a low tissue-irritating potential at low concentrations (0.5-1%) (48). Yet, it is a potent tissue irritant in higher concentrations (2.5-5%) (26, 28, 31, 48). High concentrations should therefore be either avoided or used with great care so that no solution is dropped into the eyes of the patient or extruded beyond the apical foramen, which may cause severe tissue irritation (Clinical procedure 9.1). The risk-benefit ratio of the use of high concentrations of NaOCl can be questioned further on the basis of the limited gain in antibacterial effect found in clinical trials (6, 71).
Ethylenediaminetetraacetic acid
EDTA is a calcium binder (chelator) that aids in removal of the smear layer. The smear layer is mainly composed of dentin particles embedded in an amorphous mass of organic material that forms on the inner root canal walls during the instrumentation procedure. Sodium hypochlorite is unable to dissolve this debris, which often contains bacterial organisms. Some contend that it is advantageous to leave the smear layer intact because it acts as a physical barrier for bacteria lodged in dentinal tubules and thereby locks them in. On the other hand, the smear layer counteracts disinfectants and blocks the penetration of medicaments into the dentinal tubules. It also interferes with adhesion and penetration of root filling material. For effective removal of the smear layer after preparation and irrigation with NaOCl one may leave EDTA in the canal for approximately 1 min. Alternatively, citric acid may be used for smear layer removal.
- Use a small-diameter needle (0.4 mm/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses