Chapter 8 The Maxillofacial Prosthodontist’s Role in Postcancer Rehabilitation Using Mini Dental Implants
Outline
Treatment of patients with head and neck cancer, trauma, or developmental defects requires a multidisciplinary team approach. Depending on the trauma or disease and treatment to be rendered, this team may include a surgeon(s), prosthodontist, radiation oncologist, medical oncologist, speech and language pathologist, physical therapist, psychiatrist, nursing, and social worker. Before starting any treatment—whether a single or multiple modality of surgery, radiation, or chemotherapy—a prosthodontist trained in maxillofacial prosthetics should be consulted because each of the above will have varying degrees of impact on function and future treatment that can be rendered. Anatomical variations and changes in functional jaw movement from cancer or trauma surgery, alterations in healing capacity, and oral homeostasis after radiation therapy, and changes in the immune status, healing, and physiologic balance from chemotherapy will be accounted for by the prosthodontist in determining necessary treatment before, during, and after therapy. Prosthodontic rehabilitation of the oral cavity, specifically the use of dental implants for this patient population, has been a controversial subject. The paradigm is changing, and the use of MDIs is aiding this shift in care.
Treatment of Patients Receiving Radiation and/or Chemotherapy
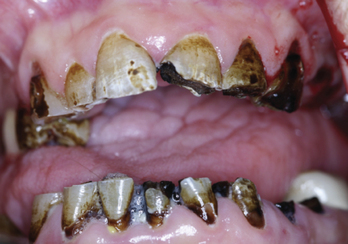
Patients who have been referred for disease management through nonsurgical means with either radiation or chemotherapy alone or combined should be seen before initiating their cancer care. A thorough examination with appropriate dental radiographs should be completed, looking specifically for teeth with a poor or hopeless prognosis. Teeth that are not able to be restored and that are in the field of radiation should be extracted as far in advance of commencement of the treatment as possible to allow for healing.11 Ideally 21 days before treatment is desirable but depending on the aggressiveness of the disease this is not always possible. The remaining teeth should be in good periodontal health and either be caries free or have sound conservative restorations. The patient should be instructed on to proper homecare regimens: brushing after every meal, flossing, daily use of a fluoride supplement such as 1.1% sodium fluoride (PreviDent 5000 Plus, Colgate Oral Pharmaceuticals, New York, New York), and seeking routine professional dental care every 3 to 4 months with a local general practitioner and hygienist for the remainder of their life.9
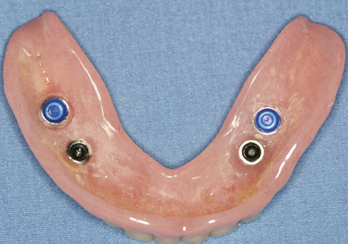
If the patient currently has MDIs or conventional implants in the field of radiation a protective mouth guard can be fabricated to lessen the effects of backscatter that cause an increase in mucositis to the adjacent soft tissues or tongue.3 The mouth guard is fabricated by making an impression with irreversible hydrocolloid, placing laboratory analogs in the impression, pouring a cast from improved stone, and using a vacuum formed material such as 0.15-inch thickness resilient material (Mouthguard Regular Clear .150, Henry Schein Inc., Melville, NY) similar to an athletic mouth guard or a combination material for a soft lined rigid protector (Proform Dual Laminates, Keystone Industries, Cherry Hill, NJ) (Figures 8-1 to 8-3). This will provide spacing between the metallic restorations and the adjacent soft tissue. It is important to note that the patient must have the mouth guard(s) in place during the simulation appointment while the positioning mask appliance is being fabricated by the radiation oncology team. Several studies have confirmed that backscatter in the bone and around the soft tissues of irradiated implants occurs; however, the negligible amount does not necessitate trephine removal of the implant.23,2
During radiation and/or chemotherapy, elective procedures should be avoided for both patient safety and comfort. The ensuing mucositis places the patient at increased risk for infection and delayed healing, and the oral cavity is generally too uncomfortable to tolerate surgical procedures. If MDI placement is deemed necessary at this time, active chemotherapy is not an absolute contraindication.14,22 Current laboratory blood counts should be obtained, paying particular attention to overall platelet count, clotting time (international normalized ratio [INR]), and immune status (specifically the absolute neutrophil count [ANC]). Because most MDIs are placed with a flapless surgical approach previously described and bleeding is minimal, a recommended platelet count of > 50,000, an INR ≤ 2.5, and an ANC >1.0 could be considered safe. The use of systemic or local antibiotics both prophylactically or adjunctly is empirical and can be used at the practitioner’s discretion (Esposito et al. 2008).8,18
After radiation and/or chemotherapy, a patient can be considered for MDI treatment for removable or fixed prosthodontics. Regarding chemotherapy, the patient should meet the criteria described above for blood counts that will allow healing properties to return to near normal even if they are on several maintenance drug therapies.15 Research and debate regarding dental implant treatment and patients using oral or intravenous (IV) bisphosphonates exists. Grant et al.10 published a review of 115 patients who were taking oral bisphosphonates and showed no deleterious effect on osseointegration or the complication of osteonecrosis of the jaw. Patients who have used or currently use IV bisphosphonates should not be considered implant candidates until further research is completed and the risks for osteonecrosis of the jaw are fully understood by patients and practitioners.21,13 The authors have successfully placed a very limited number of MDIs in oncology patients using both forms of bisphosphonate drugs, but long-term follow-up is not available, and therefore this is not recommended.
For placing MDIs in irradiated patients, several factors must be considered. The type, dose, and field of radiation play a significant role in this decision. The dose of 5500 cGy is often quoted as the cut off, presenting a relative low risk for development of osteoradionecrosis (ORN). Patients, however, must be educated about this risk, possible alteration of osseointegration, and documentation of this possibility should be made in the informed consent.17 The use of hyperbaric oxygen is controversial and debate continues today and is not advocated because current literature fails to prove its efficacy in preventing or treating ORN (Esposito et al. 2008).7,20 The flapless placement technique should lessen the risk for developing ORN because the periosteum is not manipulated to the extent as in an open procedure. Another controversial subject is the timing of implant surgery after radiation therapy. Proposed theories of bone healing alterations after radiation therapy are: decreased healing capacity is immediate and lifelong or decreased healing capacity does not occur in the first 6 months post radiation then worsens in the remaining lifespan.16 Despite the healing mechanism alterations, studies fail to show a clinically significant difference in osseointegration rates.19,6 A review of the clinical practice concurs with these published findings. When possible, implants are placed at the time of surgery, but previous radiation therapy is not an absolute contraindication. Antibiotic use is empirical, and we will routinely prescribe 0.12% chlorhexidine gluconate (PerioGard, Colgate Oral Pharmaceuticals, New York, New York) to be used twice daily for 7 to 14 days after implant placement.
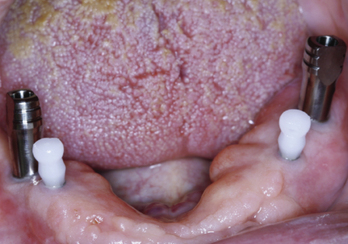
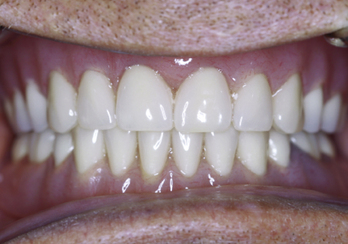
The next consideration in treatment planning implants in the irradiated patient is anatomical site. The maxillary arch has greater blood flow than the mandible. Therefore, MDIs placed in the maxilla may have a lower risk for developing ORN than those placed in the mandible. Patients who have had radiation and/or chemotherapy can be candidates for implants with proper planning and patient education. Consultation with the radiation oncologist, radiation physicist, and medical oncologist when planning MDIs for irradiated patients will provide definitive answers to the total dose, delivery method, anatomical site, and overall healing capacity of the patient, allowing for predictable and safe implant placement (Figures 8-4 to 8-7).
Treatment of Patients Before and After Surgical Resection
Hard Palate Defects
Patients that are referred before surgical resection of lesions involving only the hard palate and adjacent structures will be examined as previously described. If the patient is fully dentate then a discussion of surgical, interim, and definitive obturator prostheses procedures, expected results, and sequelae would take place. An irreversible hydrocolloid impression of the maxillary and mandibular arches is made, and a facebow recording is completed. The casts are poured in stone as a permanent record for future reference. A second impression of the maxillary arch or a duplicate of the stone cast is used to fabricate the surgical obturator. The proposed surgical resection is outlined, the tumor if visible is removed and any teeth that may be in the resection, and an acrylic resin surgical obturator is fabricated.12 This obturator is delivered in the operating room at the time of tumor resection and is held in place with orthodontic ligature wires around the remaining teeth. In approximately 10 days, the surgical obturator is removed and the interim obturator is delivered. The interim obturator is fabricated on the mounted cast that was used to fabricate the surgical obturator. If any additional teeth were removed during the surgery, they can be removed from the cast before the interim being made. Wrought wire (18 gauge) and ball clasps are contoured to the remaining teeth on the cast, denture teeth are set to the mounting, and the interim prosthesis is waxed and processed in heat polymerized acrylic resin. After removal of the surgical obturator and all remaining surgical packing, the interim obturator is fitted to place and adjusted for comfort and occlusal stability. A resilient material such as tissue conditioner (COE Comfort, GC America, Alsip, IL) is used to fabricate the obturator portion of the prosthesis. After a minimum of 3 months, longer if postoperative radiation therapy is necessary, the definitive obturator may be fabricated. The definitive obturator differs from the interim in that the healing around the defect has stabilized and a cast framework is made rather than an interim entirely from acrylic resin. The process for fabricating the cast framework closely follows procedures used for a removable partial denture framework. A diagnostic cast is surveyed for optimal placement of rest seats, placement of major/minor connectors, and development of the obturator portion of the prosthesis. The tooth preparations are completed, a master cast is made, and the laboratory fabricates the framework. After verification of the fit of the framework, a corrected cast procedure is completed to record the defect area. The trial prosthesis in wax is verified, processed, and delivered.
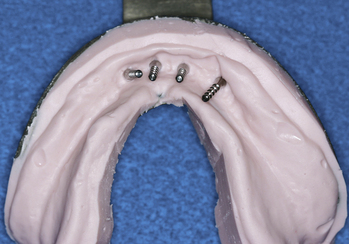
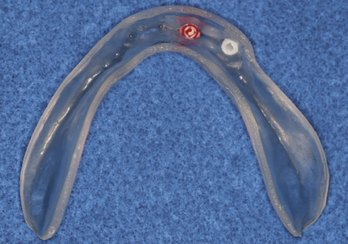
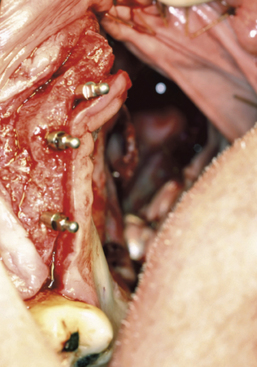
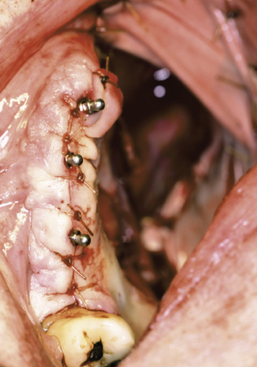
Patients who are partially or completely edentulous are faced with a greater prosthetic rehabilitation challenge due to the varying degree of stability from the lack of teeth. When evaluating these patients before the maxillectomy, discussion involving implants must be included. Although the patient may choose not to have implants placed at the time of resection, most will choose to have this done while they are under anesthesia. Additionally, educating the patient about the probable need for postoperative radiation therapy placement will allow for 6 to 8 weeks of integration and make the transition from the surgical to interim obturator easier. The procedures involved in preparing partially or completely edentulous patients closely mimic the steps describe above for dentate patients. One difference is that when fabricating the surgical obturator either a trough can be created or holes can be placed before or at the time of surgery to fit around the implants. The number of implants will vary with the anatomical volume remaining after the resection; however, all available bone should be used. The 1.2-mm osteotomy drill is used to pierce the soft tissue and just puncture the maxillary bone. The 2.4-mm MDI MAX is then allowed to self-advance through the bone using the finger, winged, and ratchet drivers until properly seated.4 If a flap is reflected, closure with sutures will complete the placement and the surgical obturator will be delivered (Figures 8-8 to 8-11). The surgical obturator is then fixed by wiring around the remaining teeth, suturing the obturator to place, using orthopedic fixation screws into the remaining palate, or transalveolar wiring. Transalveolar wiring can be accomplished using a surgical awl, sternum wiring kit, or 1-mm drill and passing the wire from buccal to lingual. After all the wires (usually 3 to 4) have been passed through, the surgical obturator can be “threaded” with the wires and seated to place. The wires are then twisted clockwise until tight and the excess cut leaving a 6-mm tail that is shaped like a rosette.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses