Chapter 8
Psychopharmacologic agents (antidepressants, antipsychotics, anxiolytics, and psychostimulants) used in chronic pain
8.1 Introduction to Psychoactive Agents and Pain
Psychoactive drugs are also described as psychopharmacological agents and they are primarily used in the treatment of mental disorders. This class of agents is used with the intent of behavior modification and alleviation of symptoms, and selection of a specific agent is based on current knowledge of the neurotransmitters, receptors, and neuronal circuits affected.1 The drugs in this classification include antidepressants, antipsychotics, anxiolytics, and psychostimulants, among others. This chapter focuses on these agents as they have been used in the comanagement of chronic pain and orofacial pain, in particular. The linkage between these drugs and their use in pain is beyond the fact that, frequently, pain patients have co-morbid depression as well as other psychosocial disorders that impact pain.2 Specifically some of the drugs discussed in this chapter are analgesic agents, which is a distinct pharmacologic property that is probably independent of their psychopharmacologic action.3,4 Moreover, these analgesic effects can be differentiated from placebo, may be seen at doses lower than those usually effective in depression, and will be seen in patients who are not depressed. Of course some of the drugs discussed in this chapter are used primarily for their psychopharmacologic effects and not for their pain effect. On the other hand, in modern clinical care, pain is considered a “vital sign” that requires routine evaluation and symptomatic treatment, and as such, the detection, quantification, and treatment of pain are also becoming part of the comprehensive psychiatric evaluation and management.5 A table listing the various medications that are considered psychopharmacologic agents and their relative importance to pain control is provided (Table 8.1).
Table 8.1 Various psychopharmacologic medications used in pain control
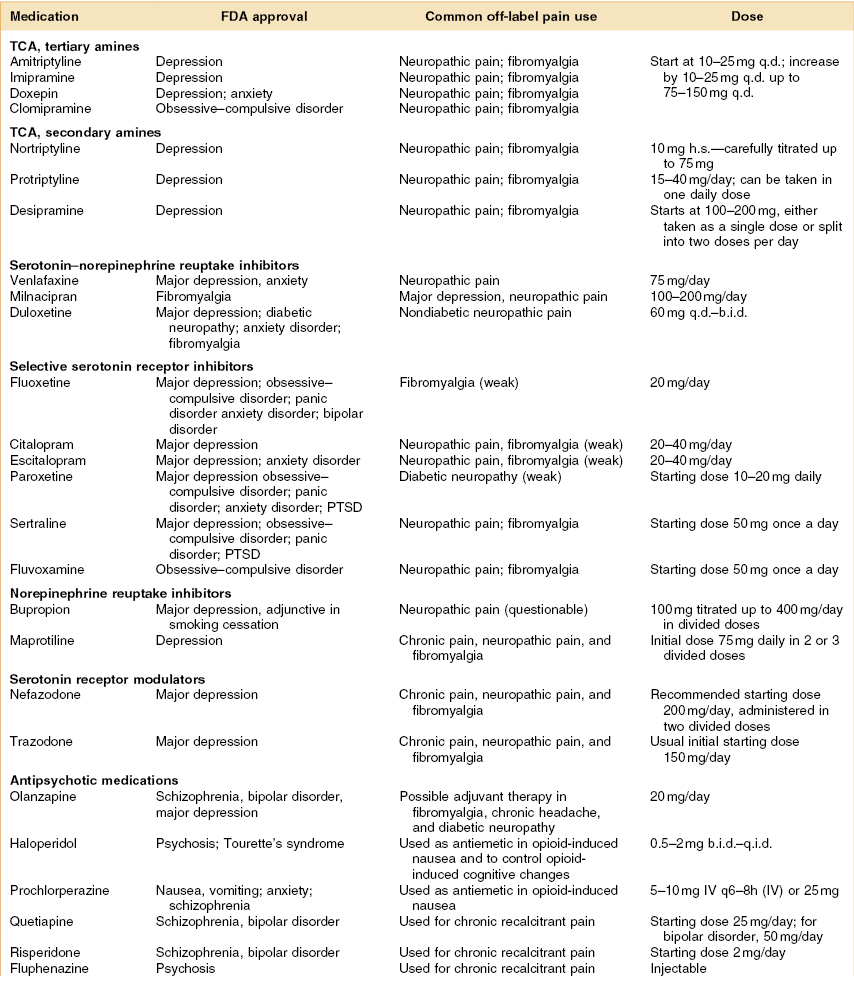
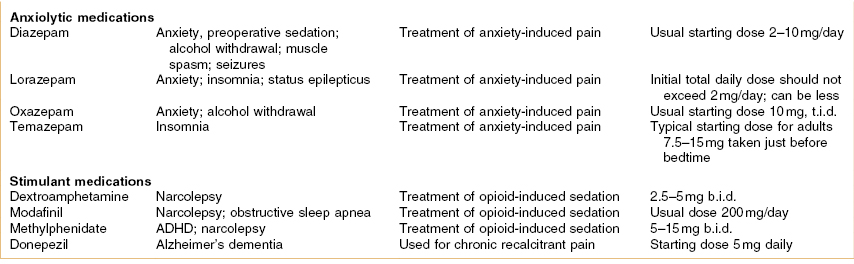
Dosing abbreviations: b.i.d., bis in die (a Latin phrase meaning “twice daily”); h.s., hora somnia (a Latin prase meaning “at bedtime”); q.d., quaque die (a Latin phrase meaning “every day”); q.i.d., quater in die (a Latin phrase meaning “four times daily”); qxh, every x hours (from quaque hora, a Latin phrase meaning “every hour”); t.i.d., ter in die (a Latin phrase meaning “three times daily”).
ADHD, attention deficit hyperactivity disorder; FDA, US Food and Drug Administration; IV, intravenous; PTSD, post-traumatic stress disorder; TCA, tricyclic antidepressant.
8.1.A Serotonin Versus Norepinephrine As a Pain Inhibitor
Several psychoactive drugs have in common the ability to inhibit the reuptake of serotonin and norepinephrine. These drugs generally do not serve as agonists to the opioid receptors, yet they are known to have analgesic properties. This suggests that blocking uptake of one or both of these neurotransmitters is important to antinociception. Codd et al.6 examined whether centrally acting analgesics (opioids) also inhibit uptake of serotonin and norepinephrine. The authors reported that certain opioids, such as morphine and naloxone, did not block norepinephrine or serotonin uptake, whereas levorphanol, levomethorphan, d-propoxyphene, and methadone did inhibit uptake. The opioids proven to block uptake also were examined to see how strong the correlation was between their antinociceptive activity and their affinity at the μ-opioid receptor (r = 0.85). The authors stated that when they considered serotonin uptake inhibiting activity (but not norepinephrine uptake inhibiting activity) this significantly improved the correlation between antinociceptive potency and the in vitro activity of these compounds (r = 0.915). The critical role of central nervous system serotonin in antinociception was confirmed by a recent series of articles which examined opioid medications in mice that were genetically engineered to lack neurons that produce serotonin.7–9 The authors found that opioids do not relieve pain as well in these genetically altered mice. Some opioids completely lost their pain-relieving effects and yet the mice still developed tolerance to the drugs and even actively sought them out. This research demonstrates that serotonin is heavily involved in antinociception. A 2004 review of pain occurring in depressive disease noted that a dysfunction of the serotonergic and noradrenergic pathways is commonly accepted as playing a major role in depression.10 These same monoamines and their neurons serve to inhibit painful stimuli coming from the intestines, the skeletal muscles, and other sensory input. Normally, these inhibitory effects are modest, but when needed, this inhibitory system may strongly suppress painful stimuli. Moreover, when a dysfunction at the level of the serotonergic and noradrenergic neurons occurs, this results in symptoms of both depression and pain.
It is important to mention that the descending spinal serotonergic pathway has an inhibitory mechanism on the primary afferent terminal via postsynaptic 5-HT1B/D receptor, but it also has a facilitatory activity on the dorsal horn excitatory 5-HT3 receptors. It has been presumed that this combination of both inhibitory and facilitatory actions of serotonin might explain why medications that increase only serotonin levels (the selective serotonin reuptake inhibitors [SSRIs]) are not as successful in the treatment of pain as drugs with actions on both serotonin and norepinephrine, which have been proven to be effective in various neuropathic pain states, such as diabetic peripheral neuropathic pain and fibromyalgia.
8.2 Antidepressants and Pain Suppression
Within the category of drugs listed as antidepressants are multiple medications and this section considers the analgesic properties of several types: the older antidepressants; the tricyclic antidepressants (TCAs); the SSRIs; and the newer serotonin and norepinephrine reuptake inhibitiors (SNRIs).11,12 Among these, the TCAs are the medication class with the strongest evidence of an analgesic effect as shown in various studies on a variety of neuropathic pain syndromes (painful diabetic neuropathy, postherpetic neuralgia, and atypical facial pain).13 In a meta-analysis, it has been shown that both TCAs and the newer SNRIs are more effective than placebo for neuropathic pain.14 This article concluded that TCAs and SNRIs have a “number needed to treat” (NNT) of approximately 3.0, which is considered fair. This means that, for every three individuals with neuropathic pain who received an optimal dose of a TCA or SNRI, at least one patient will show a moderate improvement.
Another article that examined the treatment efficacy of various antidepressant medications used to manage neuropathic pain concluded that the NNT for various TCAs ranges from 1.4 to 2.5, which is considered good. For SSRIs this article put the NNT for neuropathic pain in the 6–7 range, which is considered poor.15 Moreover, assuming these drugs are titrated to the optimal dose slowly, about 30% of the patients will have minor adverse reactions and about 1 out of 20 (5%) will have major side effects. Finally, a 2007 Cochrane Database updated report on the efficacy of antidepressants for neuropathic pain examined 61 clinical trials that tested the effect of over 20 different antidepressants.16 The review concluded that TCAs are somewhat effective for neuropathic pain and have an NNT of 3.6 for the achievement of at least moderate pain relief and that for one SNRI (venlafaxine) there were 3 studies that produced an NNT of 3.1. Of course, pain relief qualities of a medication cannot be separated from its side-effect profile, and this is rated using an NNH score (number needed to harm). For NNT the lower the number, the more effective the drug; however, for NNH the higher the number, the more people that can take the drug without side effects. NNH can be rated in two ways: the NNH for withdrawal from a study (usually due to major adverse effects) and the NNH for reporting a minor side effect but not withdrawal from the study. The NNH for major adverse effects for a commonly used TCA (amiptriptyline) was 28 and for a common SNRI (venlafaxine), 16.2. However, for an NNH defined as a minor adverse event, the NNH for amitriptyline was 6 while for venlafaxine it was 9.6.
Caution
In 2007 the FDA sent out a notice to health professionals that all antidepressant medications may increase the risks of suicidal thinking and behavior in children, adolescents, and young adults (age <25 years) during the first 1–2 months of treatment.17 This effect was not seen in adults older than 24 years of age, and adults 65 years of age and older taking antidepressants have a decreased risk of suicidality. The proposed updates apply to the entire category of antidepressants. Individuals currently taking prescribed antidepressant medications should not stop taking them and should notify their healthcare professional if they have concerns.
8.2.A Tricyclic Antidepressants
As mentioned above, there is reasonable evidence that TCAs have analgesic properties when used on a variety of chronic nonmalignant pain conditions.18–20 The mechanism of action for these drugs is that they increase the level of neurotransmitters in the central nervous system by inhibiting the reuptake of both serotonin and norepinephrine from the synaptic cleft. This class of drugs is usually separated into the tertiary amines (amitriptyline, imipramine, doxepin, clomipramine) and the secondary amines (nortriptyline, desipramine), and both are known to have antinociceptive properties. These drugs are frequently used for neuropathic pain and sometimes even for cancer pain.21–24 The main drawback for these medications is their substantial side effects, which are many—limiting their use in patients with co-morbid disease or in older adults, who are generally much less tolerant than a younger cohort.25 One of the more serious adverse effects is the potential cardiotoxicity of this class of medications.26 The general rule is that patients who have significant heart disease (conduction disorders, arrhythmias, heart failure) should not be treated with a tricyclic medication. Regarding other side effects, the secondary amine tricyclic antidepressants, desipramine and nortriptyline, are slightly less anticholinergic and, therefore, better tolerated than the tertiary amines.
These drugs have been a favorite therapy for chronic musculoskeletal pain (e.g., fibromyalgia) although they are not FDA approved for this purpose. The evidence is considered fair to good that they are better than placebo medications in randomized controlled trials on fibromyalgia.27 The advantage of these medications is that they also improve sleep and may even enhance the antinociceptive effects of NSAIDs and opioid analgesics. Most clinicians agree that when drugs in this class of antidepressants are used for pain management their analgesic effects are not due to their antidepressant effect.
Amitriptyline
Description, Mechanism of Action, and Primary Indications
The oldest and therefore the most tested drug in the TCA class, regarding its effect on pain, is amitriptyline. Laboratory studies on development and treatment of chronic pain have shown evidence that there is an interaction between tumor necrosis factor alpha (TNF-α) production and alpha(2)-adrenergic receptor response in the regulatory mechanism of neuropathic pain, which has been demonstrated to be altered by the administration of amitriptyline, inhibiting pain-induced increases in brain-associated TNF-α and transforming peripheral alpha(2)-adrenergic receptors, further regulating the production of TNF-α.28
Starting Dose
Amitriptyline should be titrated up from a typical starting dose of 10–25 mg at bedtime, and increased by 10–25 mg per week up to 75–150 mg/day.
Metabolism, Side Effects, and Adverse Drug Reactions
The main side effects are anticholinergic and can occur rapidly, although tolerance develops. Typical side effects are postural hypotension, sedation, constipation, urinary retention or frequency, weight gain, and dry mouth. Electrocardiograms should be checked in all patients over age 40. Caution is appropriate for use of amitriptyline in patients with cardiac conduction defects or arrhythmias, and in patients with narrow-angle glaucoma and benign prostate hypertrophy. It is metabolized by the liver to produce the active metabolite of nortriptyline.
Efficacy for Fibromyalgia
This drug has been evaluated in a meta-analysis29 that reviewed studies where it was used in multiple placebo-controlled trials.30–35 The largest reported result for this medication was that it clearly improved sleep quality and it had only a modest effect on palpation tenderness or muscle stiffness. One randomized blinded controlled trial examined a combination of fluoxetine (an SSRI) and amitriptyline in fibromyalgia and found them to be more effective than either agent used alone.33
Efficacy for Other Neuropathic Pain Disorders
Sharav et al.36 demonstrated that a low dose of amitriptyline (mean dose, 23.6 mg) was as effective for chronic orofacial pain as a higher dose (mean dose, 129 mg); the usual daily antidepressant dose is 150–300 mg. Another study showed that 25 mg amitriptyline daily for 3 weeks was superior to placebo in patients with chronic nonmalignant pain.37 Increasing the dose of amitriptyline to 75 mg or higher produces an improved sleep but it also causes significantly higher incidence of adverse effects.38 Amitriptyline is also known to relieve pain in nondepressed patients independent of its effect on mood alteration.39–41 Lastly, there are very few placebo-controlled studies in the literature that have examined the use of TCAs on patients with orofacial pain.42,43
Other Tricyclics—imipramine, Doxepin, Clomipramine, Nortriptyline, Protriptyline, Desipramine
Description, Mechanism of Action, and Primary Indications
See the discussion of amitriptyline (Sec. 6.2.A).
Starting Dose
The starting dose for nortriptyline is 10 mg at bedtime, and increased after 3–5 days to 20 mg at bedtime, and then carefully titrated.
Metabolism, Side Effects, and Adverse Drug Reactions
The other TCAs are useful alternatives to amitriptyline and have some small differences in their side-effect profiles and half-lives. They include imipramine, doxepin, clomipramine, nortriptyline, protriptyline, and desipramine. Most of the other TCAs have been studied for their analgesic effect compared with amitriptyline. For example, desipramine is the least anticholinergic and sedative of the TCAs. Nortriptyline is popular, maybe because it seems to be better tolerated than amitriptyline and, since it is the active metabolite of amitriptyline, it has a faster time to maximum dose.
Efficacy for Fibromyalgia
Tricyclic antidepressants such as nortriptyline and desipramine have also been demonstrated to be efficient, with some limitations, in the treatment of fibromyalgia and are still considered the most cost-effective agents for this disease. As mentioned, their analgesic effect seems to be independent of the antidepressant effect.44
Efficacy for Other Neuropathic Pain Disorders
Desipramine has also been reported to exhibit pain relief after 3 weeks, independent of mood alterations, in a placebo-controlled randomized controlled trial of 26 patients with postherpetic neuralgia.45 From 10 studies, including approximately 300 patients on active treatment, the NNT for TCAs was calculated to be 2.6. In two studies on imipramine in diabetic neuropathy, the dose was adjusted to obtain the optimal plasma concentration of imipramine plus its active metabolite desipramine, around 400 nM.46,47 The target concentration was obtained in 16 of 19 patients in the first study with a median dose of 200 mg/day (range 25–350 mg/day). From the original data of that study, an NNT of 1.4 was calculated. The literature suggests that there is no difference in NNT between TCAs with balanced reuptake of serotonin and noradrenaline (imipramine, amitriptyline, clomipramine), with NNT 2.7, and TCAs with relatively selective noradrenaline reuptake (desipramine, nortriptyline, maprotiline) with NNT 2.5, which is in accordance with one study with a face-to-face comparison,48 but in contrast to others.49,50 However, the dosage policy may be particularly important in this comparison, as relatively selective drugs are better tolerated than the balanced drugs, and the potential effect may therefore be more fully achieved for these drugs than for the balanced compounds. The data from at least two controlled studies indicate that tricyclics are effective for both steady and lancinating or brief pains,51,52 whereas it is more difficult to judge if these drugs also relieve touch-evoked pain. It is an inherited problem with these studies that none of them addressed the issue of an effect on different pain types, but only showed that patients with the different types of pain were relieved of pain in general.
8.2.B Serotonin and Norepinephrine Reuptake Inhibitors
Description, Mechanism of Action, and Primary Indications
A more recent class of antidepressants is the so-called serotonin and norepinephrine reuptake inhibitors (SNRIs). These drugs (venlafaxine, milnacipran, and duloxetine) are claimed to have lower side-effects profiles than the TCAs. A 2005 review of the SNRI medications (venlafaxine, milnacipran, and duloxetine) described that these three agents block the reuptake of both serotonin (5-HT) and norepinephrine with differing selectivity.53 The review noted that milnacipran blocks 5-HT and norepinephrine reuptake with equal affinity, duloxetine has a 10-fold selectivity for 5-HT, and venlafaxine a 30-fold selectivity for 5-HT. The review noted that these three agents are similarly efficacious for both anxiety disorders and are also helpful in relieving chronic pain (with or without depression). Unfortunately, tolerability of these three SNRIs is quite different, noting that venlafaxine seems to be the least well tolerated, while duloxetine and milnacipran appear better tolerated and essentially devoid of cardiovascular toxicity.
Starting Dose
Duloxetine is typically given in doses of 60 mg 1–2 times per day. This drug is generally well tolerated by patients.
Metabolism, Side Effects, and Adverse Drug Reactions
In a study that examined duloxetine, escitalopram, and sertraline for liver enzyme effects, duloxetine was found to have a strong metabolism-inhibitory effect on the cytochrome P450 2D6.54 This raises concerns regarding drug interactions, such as the combination of duloxetine and methadone. Side effects of nausea, dry mouth, constipation, diarrhea, and anorexia were reported more frequently with active drug than with placebo. The FDA issued a drug safety notice in 2008 stating that there have been reports of overdose with venlafaxine, occurring predominantly in combination with alcohol and/or other drugs.55 It urged healthcare professionals to prescribe this drug in the smallest quantity of capsules consistent with good patient management to reduce the risk of overdose.
Efficacy for Fibromyalgia
Duloxetine is approved by the FDA for the treatment of fibromyalgia. In one study duloxetine was given in 60 mg twice a day (b.i.d.) to 207 patients with fibromyalgia with or without current major depressive disorder.56 The duloxetine-treated patients were found to have improved significantly more on a total overall fibromyalgia questionnaire than the placebo-treated group. As a pain medication, the rationale for using these drugs in fibromyalgia is that increasing the activity of serotonin and norepinephrine may correct a functional deficit of serotonin and norepinephrine neurotransmission in the descending inhibitory pain pathways and, therefore, help reduce pain.
Efficacy for Other Neuropathic Pain Disorders
A randomized controlled trial evaluating venlafaxine showed good pain relief for painful polyneuropathy57 and for neuropathic pain following treatment of breast cancer.58
8.2.C Selective Serotonin Reuptake Inhibitors
Description, Mechanism of Action, and Primary Indications
Selective serotonin reuptake inhibitors (SSRIs) are approved for use as antidepressant drugs, but other studies have examined their use in the treatment of neuropathic pain. These drugs include fluoxetine, fluvoxamine, paroxetine, sertraline, citalopram, and escitalopram; they differ from classic TCAs in their specific inhibition of presynaptic reuptake of serotonin, but not of noradrenaline, and their lack of postsynaptic–receptor-blocking effects or quinidine-like membrane stabilization. SSRIs increase the extracellular level of serotonin in the synaptic cleft by inhibiting its uptake back into the presynaptic neuron. These drugs have a high selectivity for the serotonin transporters but a low binding affinity for the noradrenaline or dopamine transporters.
Starting Dose
Variation in dose titration of fluoxetine from 10–20 mg to 60–80 mg daily has been studied but no satisfactory long-term pain relief has been reported. The combination of fluoxetine with amitriptyline was studied in a previously referenced study by Goldenberg.33 Fluoxetine combined with cyclobenzaprine59 was also studied for efficacy in the treatment of fibromyalgia and both studies suggested that this combination approach showed better efficacy than fluoxetine alone.
Metabolism, Side Effects, and Adverse Drug Reactions
This selectivity significantly reduces the side-effects profile of SSRIs compared with the other antidepressants and reduces their risk of an interaction with other drugs, especially sedatives, antiarrhythmics, and sympathomimetics.60,61 SSRIs (except for citalopram and escitalopram) are potent enzyme inhibitors of various CYP450 enzymes with high potential for drug–drug interactions. Moreover they can increase the concentration and side effects of many drugs metabolized by the CYP450 enzymes. One major issue with SSRIs medication is that they produce the side effect of increased clenching and bruxism when used in the higher dose range.62–65 The term “SSRI-induced bruxism” has been used incorrectly to describe this condition; instead, the induced motor behavior is most likely an increased, sustained, nonspecific activation of the jaw and tongue musculature. Patients report an elevated headache and tightness in their jaw, tongue, and facial structures. This topic is discussed in more detail in Chapter 19. Only case-based literature exists at this time and further research is needed in order to define prevalence and risk factors and to establish a causal relationship between SSRI use and jaw motor hyperactivity.
Efficacy for Fibromyalgia
Overall, the various clinical trials where an SSRI has been used on fibromyalgia patients have shown mixed to poor results, suggesting that these medications are less antinociceptive than drugs with dual effects on norepinephrine and serotonin in the relief of pain. Citalopram, which has the highest selectivity for the serotonin reuptake transporters among the SSRIs, was not effective for the treatment of fibromyalgia in two small controlled studies,66,67 or was effective but only for a short time. Fluoxetine was examined in a double-blind study on fibromyalgia and was shown to be better than placebo.68 Finally, fluoxetine has been shown to have better analgesic effect than other SSRIs, but it requires higher doses33 and has better results if combined with another drug. On the other hand, the SSRIs fluoxetine and paroxetine may have additional effects on norepinephrine at adequate doses,69,70 and have been shown to improve overall symptomatology in patients with fibromyalgia in recent studies.71,72 Paroxetine alone shows weak significant effect on pain measures.73
Efficacy for Other Neuropathic Pain Disorders
A review of 13 trials (636 patients) also revealed that SSRIs did not significantly prevent migraine headaches compared with placebo and were clearly not as effective as TCAs for treatment of tension-type headache (TTH).74 Interestingly, the use of fluoxetine (an SSRI) combined with morphine in healthy volunteers in a double-blind study resulted in 3–8% increase in analgesia to electrical tooth stimulation, and reduced some of the morphine-associated symptoms such as nausea, mood reduction, and drowsiness, without affecting plasma morphine concentrations.75 A laboratory study showed that the antinociceptive effect of fluoxetine in animals was blocked with administration of naloxone, thus making the authors suggest that the mechanism of antinociception induced by fluoxetine was related to central opioid and serotoninergic pathways.76 However, the antinociceptive property of SSRIs seen in animal studies has never been achie/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


