Chapter 19
Five oral motor disorders: habitual tooth clenching and other involuntary oral motor disorders
Multiple disorders that affect the motor system need to be considered when the trigeminal, facial, or genioglossal muscles become dysfunctional. For instance, involuntary tremor suggests Parkinson’s disease; weakness suggests Bell’s palsy or stroke-related paralysis; involuntary jaw and tongue motions suggest dystonia or dyskinesia; daily jaw pain and temple headaches suggest clenching or bruxism. The focus of this chapter is on five motor disorders: (1) tooth clenching, which is presumably a learned and voluntary behavior, and the most commonly seen involuntary orofacial motor disorder (OMD): (2) sleep bruxism; (3) focal orofacial dystonia; (4) oromandibular dyskinesia; and (5) medication-induced extrapyramidal system muscle activation.1–4 Table 19.1 provides a brief definition, the main clinical features, and management approaches for these OMDs.
Table 19.1 Five oral motor disorders
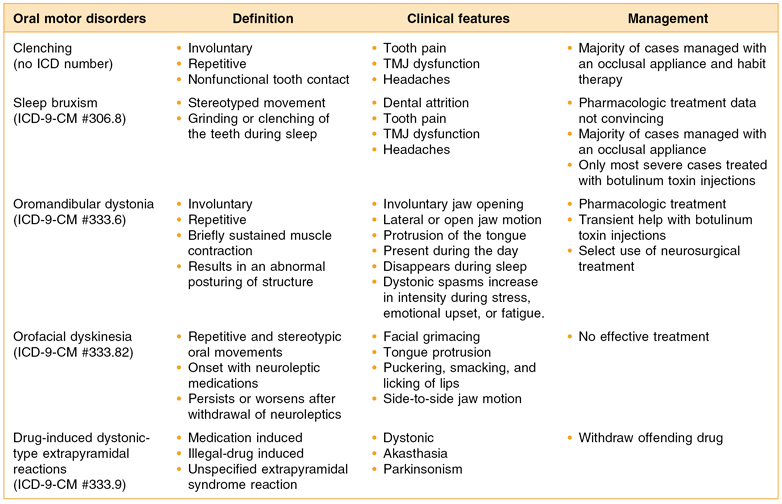
ICD, International Classification of Diseases; TMJ, temporomandibular joint.
19.1 Habitual Tooth Clenching
Many patients reporting to an orofacial pain center with a complaint of pain also report an oral parafunction such as tooth clenching, habitual cheek chewing, gum chewing, and other oral habits. In fact, sometimes patients can be seen repeatedly clenching and bulging their masseter muscles during the interview. This chapter begins with tooth clenching as it is the most common of the oral parafunctions. From a scientific perspective, the problem with tooth clenching is that little data is available on how frequently this behavior occurs in the natural environment and we do not know at what point it becomes damaging to the patient. This lack of information is because most habitual behaviors are not highly deliberate acts, but an act that someone performs at the edge of their consciousness. This means their recollection of this behavior may be inaccurate and as soon as you ask someone to self-observe how often they perform this act, they change their behavior. Alternatively, if you attach electrodes to the subject’s face and jaw muscles so that you can physically record this behavior, you also raise the subject’s awareness of the behavior and possibly change its pattern of occurrence. To accurately gather information about tooth clenching you must (1) record masseter muscle electromyograph (EMG) over a long period of time in the natural environment, (2) prove that the subject does not alter his or her behavior as a result of the recording process, and (3) you must be able to separate chewing, talking, laughing, and facial expressions, which all have a functional purpose, from those behaviors that have no obvious functional value, such as tooth clenching.
19.1.A Habitual Tooth Clenching Studies in the Natural Environment
There have been several attempts to study habitual tooth clenching in the natural environment. In the 1970s a portable EMG recording device was developed to record masseter muscle activity in the subject’s natural environment and provide auditory feedback which would let the subject know they were clenching. The first report using this device described it as a daytime use device that provided tooth clenching subjects an audio sound every time they clenched their teeth above threshold.5 This device was triggered and it recorded cumulative totals of electrical activity above a 20-µV threshold. Unfortunately, because only a single cumulative value of activity was available for each recording period, this did not distinguish between the types of oral activity the subject performed while awake (e.g., chewing, talking, laughing, clenching). This device was used almost exclusively during sleep since the other normal behaviors were less likely during sleep (see Sec. 19.2, on bruxism).
In the late 1980s and 1990s, digital technology improved dramatically, allowing better, more-detailed (second-by-second) recording of jaw muscle activity using portable equipment. One such device was developed and tested for its ability to perform long-term EMG recordings of the masseter muscle in healthy subjects in their home environment.6,7 This device was used initially to capture sleep-related jaw muscle activity and these results are described below in the section on bruxism. Several years later this device was used to examine the habitual daily masseter muscle activity of short-face versus long-face subjects as assessed in their natural environment and the data reported on how much, how often, and how long normal nonpain subjects clench their teeth.8 Specifically the study described 14 long-face and 16 short-face subjects who were selected for long-term masseter muscle activity monitoring (8 hours per day) in the natural environment over three working days. The data outcomes included the number of activity periods per hour above threshold and the mean amplitude and duration of these periods. The authors reported that the maximal EMG activities seen with experimental clenching did not differ significantly between the short-face and the long-face subjects and they also reported no significant difference between the two groups regarding the amplitude and duration of activity seen in the natural environment. The mean duration for each activity period was also not different by group and averaged 4.2 seconds in duration. What was not known is how often, how long, and how much do patients with facial muscle pain clench their teeth.
Using a somewhat similar recording apparatus ambulatory daytime and sleep period jaw motor activity was recorded bilaterally from 15 young adult patients with a clear-cut skeletal jaw deformity and 15 healthy controls (without jaw deformity).9 The surface EMG activities were averaged, rectified, and normalized to a reference task which involved a 98-N bite force. The focus was on bilateral symmetry of right and left masseter and anterior temporal muscles in the controls and the craniofacial deformity patients. The authors showed a 15–30% right-versus-left side EMG signal difference in their controls during the day, chewing, and even when sleeping. The control subject data showed that daytime nonchewing activity (presumably clenching, talking, etc.) was 5–9 times lower than the level of jaw muscle activity generated during chewing and just slightly lower than the sleeping masticatory activity levels being generated. Again no data is available on the frequency, duration, or amplitude of jaw muscle activity in myogenous-pain-reporting subjects who self-acknowledge “clenching” behaviors.
19.1.B Habitual Tooth Clenching Studies in the Laboratory
Kato et al. (2006) gathered the frequency of spontaneous functional and nonfunctional orofacial activities (under direct observation) in subjects without pain under laboratory conditions.10 Sixteen asymptomatic subjects who were instructed to read silently for 30 minutes while polygraphic recordings (including audio–video monitoring) were made of their jaw and facial muscles. Orofacial behaviors were scored based on the polygraphic and audio–video records and the magnitude and duration of masseter EMG bursts were calculated for each behavior. The number of orofacial behaviors varied widely between subjects, and the most frequently observed behavior was swallowing. Approximately half of the orofacial behaviors that occurred were closely associated with body movements but 45% of all masseter muscle EMG bursts was regarded as nonfunctional activity. More than 80% of these masseter bursts were <2 seconds in duration and had an amplitude of less than 20% of maximal voluntary contraction (MVC). Overall, the authors concluded that spontaneous orofacial motor activity is variable and even asymptomatic subjects can exhibit substantial masseter bursts during wakefulness that are not associated with purposeful, function-driven activity. Unfortunately, a comparison group of subjects with facial pain of myogenous origin was not available.
19.1.C Self-Reported Frequency of Habitual Tooth Clenching
There are several studies in the literature which have described the frequency of self-reported clenching events in healthy controls and in patients with facial pain. For instance, Glaros et al. (2005) reported on the number of times during the day normal subjects and facial pain subjects self-reported they had their teeth in contact.11 Specifically this study included 3 groups of temporomandibular dysfunction (TMD) patients and a group of normal controls who were asked to carry pagers for one week. Subjects were contacted approximately every two hours by an automated calling system; when contacted they would complete a questionnaire assessing if they had tooth contact, facial tension, and facial–jaw pain. Results showed that patients with myofascial pain with or without arthralgia reported more frequent tooth contact, higher intensity contact, and more tension than patients with a TMJ disk displacement or the normal controls.
Asking the same question, Chen et al. (2007) examined the number and frequency of nonfunctional tooth contacts in healthy controls and patients with myogenous-based facial pain.12 Specifically the study was performed on 24 subjects (15 controls and 9 patients with myogenous facial pain). Data on tooth clenching was gathered for more days and at more frequent intervals than the previously described study by Glaros et al. (2005). Recordings were made for 10 days the subjects were alerted by a radio-wave-activated wrist vibrator approximately every 20 minutes during the day to report whether the teeth were in contact. Subjects also completed two stress assessment questionnaires that were designed to measure perceived stress levels. Similar to the earlier study, these authors also reported that there was a significantly higher frequency (more than four times) of wake-time nonfunctional tooth contact in the myogenous pain patients than in controls (median of 34.9% versus 8.9%) of the day. In both groups the frequency of nonfunctional tooth contact did not significantly differ among the various days or between the genders. The pain patients had significantly higher stress scores and reported having experienced more stressful situations than the controls. The above two studies suggest that increased masticatory muscle activity responsible for tooth contact may be an important mechanism in the etiology of myofascial pain and arthralgia of TMJ.
19.1.D Harmfulness of Habitual Tooth Clenching
Certainly the data above suggests that putting your teeth together with a sustained low-level force is not unusual and in most cases it is also not harmful to the jaw system. The question that must be answered is, “When does clenching turn harmful?” This question has also received moderate attention by researchers over the last few years. Widmalm et al. (1995) examined the relationship between oral parafunctions and jaw pain or dysfunction using data gathered by both questionnaire and examination.13 The study sample included 203 children between the ages of 4 and 6 years. The authors found statistically significant correlations between reported bruxism, nail biting, thumb sucking, and most of the signs and symptoms associated with TMDs. A significant relationship between oral parafunction, temporomandibular disorders, and emotional status was observed in a similar study of 502 children between the ages of 3 and 7 years.14 These authors gathered information (both subjective and objective) about the signs and symptoms of temporomandibular disorders, including attrition, oral parafunction, and emotional status in preschool children which was confirmed by parental questionnaire. The results showed significant associations between (1) attrition and emotional status, as well as several temporomandibular joint symptoms and (2) the reported habit of tooth grinding and jaw pain. A serious limitation of the above two studies is the age of the subjects since self-report of bruxism, facial pain, and clinical examination is not of proven reliability in children between 3 and 7 years of age.
There are several studies on adults which have examined whether oral habits are related to TMD. Moss et al. (1995) used self-rating of oral habits over a 7-day period in subjects with and without facial pain.15 The results indicated a significant relationship between TMD and self-reported teeth clenching. As with the two studies on children, there was a statistically significant association between pain status and TMD. Glaros et al. (2005) examined the role of parafunctions, emotions and stress in predicting facial pain in 96 subjects who had been diagnosed with either (1) myofascial pain, (2) myofascial pain and arthralgia, (3) disk displacement, or (4) a healthy control group.16 The authors asked patients to record their pain, current activity/behavior and emotional level each time they were paged (approximately every two hours) during their waking hours. The authors reported that two myofascial pain groups scored higher than did the groups with disk displacement or controls on the following: pain; masticatory muscle tension; and a composite variable measuring time and intensity of contact; mood; and stress. A linear regression analysis showed that masticatory muscle tension and the composite variable was able to predict jaw pain and accounted for 69% of the variance in jaw pain.
One must be skeptical when reading studies that collect data on parafunctional habits of children or adults based on self-reporting or parental reports and not on direct measurement of jaw function. Even if the study can show an association between myogenous pain and self-reported oral parafunctions, this does not prove causality. Nevertheless, the conclusion derived from the above studies suggest that indeed parafunctional behaviors, especially those that are associated with emotional states, increased muscle tension, and sustained tooth clenching can be harmful in patients with myogenous facial pain.
19.1.E Experimental Tooth Clenching, Injected Algesic Agents, and Jaw Pain
Several researchers have examined the question, “Does experimental clenching actually produce sustained pain in the jaw?” The approach used was based on the idea that if tooth clenching is harmful, then it should be possible to experimentally produce pain in patients by having them mimic this behavior. Several prior studies have looked at jaw clenching exercise, endurance, and postexercise pain and recovery of maximum bite force ability following exercise. Specifically, the studies reported the effect of repeated sustained isometric contractions of the jaw-closing and protrusive muscles on jaw pain and stiffness during the week following the experiment.17,18 These two studies examined the pressure pain threshold of masticatory muscles, maximum active pain-free jaw opening, and overall jaw pain level before, during, immediately after, and 1, 2, 3, and 7 days after higher force muscle sustained contraction tasks were performed by healthy male volunteers (aged 24–39 years). They reported that jaw pain level significantly increased during and immediately (1 hour) after the experimental task. However, these subjects demonstrated only very low levels of postexperimental jaw muscle pain and soreness at 1, 2, and 3 days after the contraction tasks. This result was surprising given that it is common to induce postexercise muscle soreness in limb muscles with sustained exercise. The study did not include women in the experiment. One report in the literature that did include women in a postexercise jaw-muscle soreness study included 7 male and 7 female healthy subjects (aged 25 ± 3 years) who performed various intermittent and sustained high-force contraction tasks.19 The main difference with the Plesh et al. (1998) study and the prior two studies by Clark et al. (1989, 1991) was that there was far more postexercise overall jaw pain level on the first and second days after the exercise, and a significant decrease in pain-free jaw opening distance on the second day in females than in males.
Svensson et al. (2001) tried to induce fatigue and pain in the jaw motor system by using a lower level sustained-force clenching task.20 Eleven healthy men were asked to clench on a bite-force meter for 60 minutes at 10% of the maximum voluntary contraction (MVC). The authors described that all participants reported an increasing sensation of jaw fatigue and 7 out of 11 reported mild facial pain in the jaw-closing muscles during the task but all were able to maintain the required force without drop-off. Unfortunately, they did not assess if the pain lasted for any substantial length of time beyond the actual experimental task and they did not include females in the study sample. Torisu et al. (2006) conducted a similar type of study using a low-level experimental sustained clenching task to see its effect on muscle pain, fatigue, and resting jaw-muscle activity in 12 healthy men and 11 healthy women.21 This experiment supplemented the change induced by the low-level tooth-clenching with an injection of glutamate (an algesic substance) at the end of the clenching task. Pain and EMG data were recorded before and at 3 points after a 30 minute tooth-clenching task at 10% of maximal force. The first postclenching data was preinjection (Post-1), the second postclenching data (Post-2) was after a glutamate (Glu) or isotonic saline (Iso) injection into the left masseter, and the last data set (Post-3) was collected at 60 minutes after the end of the tooth-clenching task. The authors reported that sustained low-level tooth-clenching consistently produced some muscle pain and headache-like symptoms in both genders. The authors found that there was a larger increase in the resting EMG activity in women than in men in the masseter muscles, suggesting more pain-induced muscle-guarding behavior in the female subjects. Finally, there was no significant difference by gender for the perceived amount of experimental pain induced by the glutamate injection. Again proof of long-lasting pain was not provided by this experiment.
19.2 Bruxism
Sleep bruxism (SB) is a stereotyped movement disorder characterized by grinding or clenching of the teeth during sleep. This definition is from the American Academy of Sleep Medicine Classification of Sleep Disorders, which considers clenching a part of sleep bruxism. It involves strong contractions of the jaw muscles during sleep; these contractions can be rhythmical or continuous isometric contractions lasting from several seconds to as much as 10 minutes each night.22 Bruxism commonly involves two basic patterns: (1) rhythmic, chewinglike movements and (2) prolonged, maximal isotonic contractions of the jaw muscles. Periods of sustained contractions have been observed to continue for up to 300 seconds. Abnormal wear to the teeth is the most often mentioned clinical sign of bruxism and is often used to determine whether a subject is a strong bruxer or not. Unfortunately, wear of the teeth is not a good measure of active ongoing bruxism and may be moderately influenced by the amount of saliva the subject produces during sleep.23
19.2.A Prevalence of Bruxism
The prevalence of chronic bruxism is unknown because no large probability-based random sample study has been performed using polysomnography (PSG), which is needed to measure bruxism. The prevalence in the general adult population has been reported to be between 3% and 90% and, among children, prevalence ranges from 7% to 88%.24–32 Of course, many bruxers do not have substantial attrition and many also do not make tooth grinding sounds during sleep, so sleep partners or parental reports are not always accurate. However, it is not clear that bruxing among children is the same problem as in adults.33,34 In younger children, bruxism may be a consequence of the immaturity of the masticatory neuromuscular system. The complications of bruxism reported in children include dental attrition, headaches, and masticatory muscle soreness.35 Sleep bruxism in children has been shown to occur more frequently in stage 2 and rapid-eye-movement (REM) sleep, with arousals in 66% of the cases. The high arousal index in children with bruxism has been moderately correlated with increased incidence of attention–behavior problems.36 Children who brux also have high-anxiety-prone personalities.37
19.2.B Pathophysiology of Bruxism
The pathophysiology of bruxism is unknown. Various factors have been associated with bruxism. The most cogent theories describe bruxism as a neuromotor dysregulation disorder. This theory proposes that bruxism occurs due to the failure to inhibit jaw motor activity during a sleep state arousal. There are numerous clear-cut neuromotor diseases that exhibit bruxism as a feature of the disease (e.g., cerebral palsy). The disorder of periodic limb movements is thought to be quite similar to bruxism except that it occurs in the leg muscles rather than in the jaw.38 Bruxism has been reported during each stage of sleep; however, the majority of episodes appear during stage 2 sleep.39,40 Bruxism also occurs frequently when the patient moves from a deeper to a lighter stage of sleep and can be induced by attempts at waking the sleeping subject.41 Consequently, some bruxism episodes appear to be part of an arousal phenomenon including an increase in heart rate and respiration, galvanic skin resistance changes, and the appearance of the K-complex on the electroencephalogram. Although most bruxism episodes appear to occur during stage 2 sleep and during arousal, others have reported that bruxism may occur during REM sleep.42–44
Although, patients with SB show a higher incidence of rhythmic masticatory muscle activity (RMMA) during sleep than matched normal controls, they are good sleepers. Sleep macrostructure (e.g., total sleep time, sleep latency, number of awakenings or sleep stage shifts, and sleep stage duration) is similar between groups. Differences in sleep microstructure between SB patients and normals have been investigated in only a few studies. Lavigne et al. (2002) quantified the number of microarousals, K-complexes, K-alphas, EEG spindles, and the density of slow wave activity, in both bruxers and control groups, in order to better understand the pathophysiology of SB. SB patients showed 6 times more RMMA episodes per hour of sleep than controls, with a higher frequency in the second and third non-REM to REM cycles. SB patients presented 42.7% fewer K-complexes per hour of stage 2 sleep, but only normals showed a decline from the first to fourth non-REM episode. Only 24% of SB–RMMA episodes were associated with K-complexes in 60 seconds. The number of K-alphas was 61% lower in SB patients, no change across non-REM episodes was noted. While no difference in electroencephalographic (EEG) spindles or slow wave activity (SWA) was observed between groups, EEG spindles increased and SWA decreased linearly over consecutive non-REM to REM cycles. The authors concluded that good sleep in SB patients is characterized by a low incidence of K-complexes or K-alphas and by the absence of any difference in other sleep microstructure variables or sleep wave activity.45 In 2006, a study showed a shift in sympatho-vagal balance toward increased sympathetic activity started 8 minutes preceding SB onset. In moderate to severe SB subjects, a clear increase in sympathetic activity precedes SB onset.46 Moreover, the onset of RMMA and SB episodes during sleep were shown to be under the influences of brief and transient activity of the brainstem arousal–reticular ascending system contributing to the increase of activity in autonomic–cardiac and motor modulatory networks.47
19.2.C Actual Frequency, Duration, and Amplitude of Sleep Bruxism
Data is available from the early single-channel EMG recorders that captured cumulative nocturnal masseter muscle activity, which was presumably due to bruxism.48–51 The disadvantages of using this method were that second-by-second levels of muscle activity during sleep were not available and correlations between motor activity and sleep status were not possible. Nevertheless, these single-channel EMG–based home measurement systems have helped researchers achieve a greater understanding of bruxism. Since then several studies have examined what are the correct features of a masseter muscle EMG activity recording that define bruxism (e.g., EMG amplitude, rhythmicity, and duration).52–54 Gallo et al. (1999) used their long-term sleep EMG recording device to record masseter muscle activity in healthy subjects in their home environment.55 The study was performed on 21 healthy subjects selected after telephone and questionnaire screenings and clinical examination from among randomly selected inhabitants of Zürich. The masseter EMG was recorded across seven nights in each subject’s natural environment. The signal was analyzed for number, amplitude, and duration of contraction periods. The signal amplitude was expressed as a percentage of the amplitude recorded during maximum voluntary contractions (%MVC) and it was determined that an average of 10.5 ± 3.8 per hour contraction episodes above threshold occurred for all subjects with men having more episodes than women. They also reported that the average mean amplitude was 26.2 ± 6.4% of MVC again with men having a consistently high level of contraction than women. Finally, the duration of the episodes had a mode of 0.5 second. They concluded that healthy subjects showed intermittent periods of masseter activity during sleep which, on average, were of rather low intensity and short duration. This finding was in agreement with data provided by Nishigawa et al. (2001), who measured bruxism forces using strain gauge tranducers in occlusal splints during sleep in known bruxers.56 In their study, the mean amplitude of all bruxism events was 22.5 kgf (kilogram force) and the duration was 7.1 seconds. The highest amplitude of nocturnal bite force in individual subjects was 42.3 kgf (15.6 ± 81.2 kgf). Maximum voluntary bite force during the daytime was 79.0 kgf (51.8 ± 99.7 kgf) and the mean ratio of nocturnal to daytime maximum bite force was 53.1%. These data indicate that nocturnal bite force during bruxism can exceed the amplitude of maximum voluntary bite force during the daytime.
In 2003, Baba et al. described a device which recorded tooth contact patterns on an occlusal splint using a piezoelectric film embedded in the splint.57 Specifically, the study examined the reliability and utility of an intrasplint, force-based, bruxism detection system for multiple night recordings of forceful tooth-to-splint contacts in sleeping human subjects in their home environment. Bruxism type forces, i.e., forceful tooth-to-splint contacts, during the night were recorded with this system in 12 subjects (6 bruxers and 6 controls) for 5 nights in their home environment; a laboratory-based nocturnal polysomnogram (NPSG) study was also performed on one of these subjects. All 12 subjects were able to use the device without substantial difficulty on a nightly basis. The bruxer group exhibited bruxism events of significantly longer duration than the control group (27 s/h vs. 7.4 s/h). The PSG study performed on 1 subject revealed that, when the masseter muscle EMG was used as a “gold standard,” the intrasplint force detector system had a sensitivity of 0.89. The correlation coefficient between the duration of events detected by the force based system and the EMG method was also 0.89. Watanabe et al. (2003) described the frequency of bruxism levels and examined if this behavior was being influenced by daily behaviors from daily diary data and from tooth contact recordings made over a 3-week period using the intrasplint force detection system described previously.58 Specifically, the study included 12 patients (6 females and 6 males) with a sleep bruxism disorder to see if any daily behaviors (stress, physical activity, anger), jaw-pain/headache symptoms, or sleep quality were correlated with their sleep bruxism levels. Bruxism was defined as a force applied to the occlusal surface of the splint at or above a level of 10% MVC. VAS scales were used by the subjects to rate their daily behaviors, sleep quality, and jaw-pain/headache symptoms in a diary. The authors reported that bruxism and sleep disturbance, and the mean bruxism score for the male subjects was significantly higher than that seen for the female subjects. Overall, no single daily diary variable was consistently correlated with the bruxism levels in these subjects. They concluded that bruxism is not strongly related to any of the subject’s self-monitored daytime activities or sleep quality.
19.2.D Harmfulness of Bruxism
Sleep-state motor behaviors such as bruxism have been suggested as an important etiology causing TMD, pain and morning-onset tension-type headache. Bruxism is a potential contributor to the cause of both tension-type headaches and temporomandibular disorders. Clark et al. (1981) conducted one of the first studies which examined an association between nocturnal masseter muscle activity and severity of myofascial pain symptoms.59 Specifically, the study evaluated the level of nocturnal masseter activity and the symptoms of jaw dysfunction in 85 subjects who varied with respect to degree of jaw dysfunction. A combined jaw dysfunction index was employed which evaluated both the patients’ subjective report of pain as well as the clinical examination evidence of jaw dysfunction. Using this combined index, a significant correlation was found between the level of nocturnal masseter activity and the signs and symptoms of jaw dysfunction. Molina et al. (1999) examined the relationship of bruxism (based on subjective reports by the patient) and TMJ signs and symptoms. The study reported 207 patients who were subdivided into mild, moderate, and severe bruxism categories using a questionnaire and clinical examination. They found the smallest mean jaw opening (39.2 mm) and the highest prevalence of capsulitis (97.8%) in the severe bruxism patients. They concluded that that severe bruxers are more impaired by muscular and joint disorders as compared to mild and moderate bruxers.60
Many clinicians feel that nocturnal bruxism is partially responsible for many of the spontaneous onset TMJ internal derangements but few studies have actually measured this behavior in any detail. One notable study collected EMG based recordings for 6 nights on 103 young adult subjects (age range, 22–32 years) who also had a careful examination of the jaw for signs and symptoms of temporomandibular disorders.61 The EMG data were considered dependent variables, while the questionnaire and examination data were considered independent variables. Multiple stepwise linear regression analysis was utilized and TMJ sound scores were found to be significantly related to the duration of EMG activity (i.e., the longer the EMG duration, the more likely the subject would exhibit joint sounds). None of the other independent variables were found to be related to any of the muscle activity variables.
19.2.E Treatment Methods for Bruxism
Occlusal Appliances for Bruxism
The primary management method/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


