CHAPTER 8 EPIDEMIOLOGY OF ORAL DISEASES
IMPORTANCE OF ORAL EPIDEMIOLOGY
Awareness by the practitioner of the epidemiology of oral diseases can be of broader benefit to society. The practitioner may be called on to be a source of expert guidance to the community on public health matters related to oral health. Only by having an understanding of the distribution and determinants of oral disease within populations and subgroups of the population, along with knowledge of risk and preventive factors, will the oral health practitioner be able to provide that expertise. In addition, the alert practitioner with an understanding of the epidemiology of oral diseases may be the first to identify the presence in the population of an unusual pattern of disease. The result of this can be profound. A classic example is Frederick McKay, who shortly after graduation from dental school called attention to the presence of an unusually high prevalence of enamel staining among his patients in Colorado Springs.1 His further observation that these same patients had a lower caries prevalence led ultimately to the discovery that optimally fluoridated drinking water could dramatically reduce the incidence of caries, arguably the most important discovery made in the history of dental public health.
INTRODUCTION TO EPIDEMIOLOGY
Epidemiology is a discipline that strives to understand the occurrence of disease among groups of people. One of the more widely cited definitions defines epidemiology as “the study of the distribution and determinants of disease frequency in man.”2 Oral epidemiology is the application of epidemiologic principles to the study of oral diseases.
Epidemiologic investigations usually measure and report findings in terms of either the prevalence or incidence of disease. The prevalence of disease is the proportion of existing cases of a disease in a population at one point in time or during a specified period of time.3 For example, one might report a caries prevalence of 20% in a population of adults, meaning that 20% of the population had existing caries at a particular point or period in time (e.g., spring 2001). The incidence of disease is the number of new cases of a disease that occur in a population at risk for the disease during a specific time period (e.g., 1 year or a period of years).3 For example, one might report the incidence of caries in a population to be 20% per year, meaning that 20% of the population at risk for caries developed new caries during a 1-year period. Edentulous people in the population would not be part of this incidence calculation because these individuals, lacking any teeth, would not be at risk for caries. Sometimes the population at risk and time period are combined into a single measure of person-time (e.g., person-years).3
Epidemiologic Studies that Test Hypotheses
Observational studies attempt to assess the relationship between exposures and disease by observing exposure-disease associations as they naturally occur in the population under study. This exposure-disease relationship is usually reported in terms of an estimate of the relative risk of disease associated with a particular exposure. The relative risk can be defined as follows:3
Because the relative risk is a ratio, a relative risk of 1.0 (i.e., a 1:1 ratio) represents no difference in risk of disease associated with an exposure. Relative risk estimates above 1.0 suggest increased risk of disease associated with the exposure, whereas relative risk estimates between 0 and 1.0 suggest a protective effect associated with the exposure. Because of the potential for bias and general noise in the data that inherently exists in epidemiologic studies (as contrasted with many laboratory studies), generally relative risk estimates that lie in the ranges of 0.7–1.0 and 1.0–1.5 are considered to be weak evidence of an association.4 Generally speaking, relative risk estimates of greater than 3.0 or less than 0.3 are considered estimates of a strong association (in the direction of increased risk or protective effect, respectively).4 Because many diseases may be associated with multiple exposures or other factors (e.g., age), some of which may be related to one another, it is important for risk factor studies to use study designs and statistical techniques that allow for a simultaneous assessment of the relationship between the disease of interest and these multiple exposures or factors.
The Cross-Sectional Study
Consider the hypothesis that the presence of a restoration near the gingival margin increases the risk of developing gingival inflammation (gingivitis) among adolescents. If researchers chose to conduct a cross-sectional study to explore this hypothesis, they might examine a group of adolescents for restorations at the gingival margin and then compare the occurrence of gingivitis among adolescents with and without these restorations. The researchers could then determine if there was an association between the presence of restorations and the presence of gingivitis. Although the cross-sectional study is relatively quick and inexpensive to do, its potential to contribute to a judgment of causation is limited because it cannot determine whether the exposure of interest (in this example, a restoration at the gingival margin) occurred before the disease of interest (gingivitis). For example, the argument could be made that adolescents with gingivitis might be less likely to brush because of gingival bleeding and sensitivity and therefore are at greater risk of developing caries requiring restoration near the gingival margin, subsequent to the presence of the gingivitis.
The Case-Control Study
If the researchers had instead chosen to conduct a case-control study to explore this same hypothesis, subjects would have been split into two groups, those with gingivitis and those without, based on an examination. To search for an association with restorations at the gingival margin, a history of restoration placement at the gingival margin before the occurrence of gingivitis would be sought, for example, through past dental records. Thus the case-control study could establish a temporal relationship between the exposure and disease of interest, in this case a history of restoration placement before the occurrence of gingivitis. This type of study is only as good as the techniques it uses to gain a good history of exposure. Also, because a case-control design ordinarily does not allow direct measurement of incidence rates, it must estimate the relative risk using a measure called the odds ratio, a valid, but indirect, estimator of the relative risk.5
The Community Trial
Community trials are conducted in situations where an intervention can be practically evaluated only at the community level. In these studies, treatment is assigned on the basis of the community rather than the individual. The more that the comparison communities are similar in all aspects except the intervention under study, the more valid this type of study will be. The Newburgh-Kingston water fluoridation trial is a classic example of a community trial.6
Causation
Although an epidemiologic study might identify an association between an exposure and a disease, that is not the same thing as “proving” that the exposure caused the disease. Demonstration of an association only indicates that there is some relationship between the exposure and the disease, of which one possibility is a causal relationship. In fact, no epidemiologic study can ever “prove” causation. Causation is a judgment that can only be arrived at following a consideration of all available evidence.3 Over the years, criteria have been developed to aid in the process of arriving at a judgment of causation. The first criterion is the demonstration of a temporal relationship (i.e., it is established that the exposure of interest has preceded the disease in time). The second criterion when arriving at a judgment of causation is the strength of association (measured as relative risk); the stronger the association, the less likely the association is due to some other biasing factor. However, this criterion does not mean that a weak association cannot be causal. The third criterion is consistency of findings reported from different investigations on different populations. The greater the number of different studies showing a particular association, the less likely the association is due to some bias or deficiency in study design, because it becomes increasingly less likely that all of the studies would share the same particular bias or deficiency. A judgment of causation is further supported if the finding is coherent with existing knowledge, although it should be realized that “existing knowledge” is continuously changing. There is no rule as to when it is appropriate for a judgment of causation to be made. Consideration of the consequences of accepting a judgment of causation balanced against the consequences of delaying acceptance is often part of the process.
EPIDEMIOLOGY OF DENTAL CARIES
The Measurement Of Caries
Caries is the pathologic process of localized destruction of tooth tissues by microorganisms.7 Dental caries can be described epidemiologically in several useful ways, each of which helps our understanding of caries activity within groups of people. The conventional method of defining dental caries in a population is to measure either the number of teeth or the number of tooth surfaces that are decayed, missing, or filled as a result of caries. When this measure is applied to the permanent dentition, the acronyms DMFT and DMFS are used, indicating the number of decayed, missing, or filled teeth (DMFT) or decayed, missing, or filled surfaces (DMFS).8 When this measure is applied to the primary dentition, the acronyms deft and defs are used, with e indicating a carious primary tooth that is indicated for extraction.9 Because of confusion over how this “e” term should be interpreted and applied, recent surveys often simply report decayed or filled teeth (dft) or decayed or filled surfaces (dfs). Measuring caries by surfaces affected (i.e., the DMFS or DFS) is more precise than measuring caries by affected teeth because, for example, a tooth with five surfaces affected by caries would make the same contribution to the DMFT score as a tooth with only one affected surface. At the same time, however, the DMFT can be a useful public health measure because it is an indicator of the number of teeth that have had or require treatment. The missing teeth component of the DMFT and DMFS assumes that missing teeth have been lost because of caries. Although this assumption is reasonable for children, among adults, the older the population, the more likely it becomes that teeth may be lost as a result of other causes, for example, periodontal disease. For this reason a DFS is often used to measure caries in adult populations. Although this measure may underestimate total caries experience (because teeth missing as a result of caries are not included), it avoids a biased overestimate that would result from including missing teeth that were not lost because of caries.
A second important way to specify caries activity is on the basis of the type of tooth surface that has been affected by decay. Thus the presence of caries in a population can be described in terms of coronal pit and fissure caries, coronal smooth surface caries, and root surface caries. Describing caries in this surface-specific way can provide greater insights into potential risk factors and allow for more effective planning of preventive strategies.
Caries in Children
Several demographic factors have been consistently shown to be related to the occurrence of caries. These factors are associated with either the person or the environment in which that person has lived. As with many diseases, age is directly and strongly associated with the prevalence of dental caries. The relationship between age and caries is illustrated in Fig. 8-1, drawn from two National Institute of Dental and Craniofacial Research (NIDCR) surveys of the oral health of U.S. children and adults.10,11 One can see that with increasing age the number of surfaces affected by caries increases, plateauing at around 50 years of age. This figure also illustrates the relationship between caries and gender. One can see that the mean DMFS for males tends to lag behind that of females. For example, 14-year-old boys have approximately the same mean DMFS as 13-year-old girls (i.e., 4.2 mean DMF surfaces). One explanation for this finding is the parallel finding that the dentition appears to erupt earlier in girls.12,13 Thus at any given age the dentition in males has been at risk for a shorter period of time as compared with females.
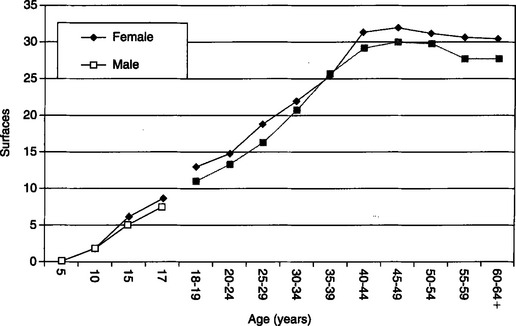
Fig. 8-1 Relationship between age, gender, and mean DMFS among U.S. children and mean DFS among U.S. adults.
(Modified from US Department of Health and Human Services: Oral health of United States children: the National Survey of Dental Caries in U.S. School Children: 1986-1987, National Institutes of Health pub no 89-2247, Washington, DC, 1989, US Government Printing Office; US Department of Health and Human Services: Oral health of United States adults: the National Survey of Oral Health in U.S. Employed Adults and Seniors: 1985-1986, National Institutes of Health pub no 87-2868, Washington, DC, 1987, US Government Printing Office.)
The relationship between race and caries is more equivocal. Although in the past, some studies had reported a lower caries prevalence among African-American children as compared with Caucasian children, others had reported either a higher caries prevalence among African-Americans or no difference.14 Two recent national surveys in the United States indicate little difference in caries prevalence between Caucasian and African-American children.10,13 However, consistent differences in the extent to which caries has been treated were found. For example, the NHANES III survey reported that although the mean DMFS for Caucasian and African-American children was virtually identical, untreated decay was more than twice as high among African-American children.13
Historically, the prevalence of caries in the United States has varied by geographic region of the country. This finding can be traced back to the time of the Civil War and continues today.14 The advent of artificial water fluoridation has had a profound effect on the distribution of caries. Historically, the prevalence of caries would be as much as 60% lower in fluoridated areas as compared with nonfluoridated areas.15 Today, this type of comparison is less meaningful, because children living in areas not served by a fluoridated water supply may be indirectly benefiting from it by drinking beverages manufactured in a fluoridated community and thus prepared with fluoridated water.16,17 National survey data show that the greater the number of fluoridated communities in a region, the less the difference in caries prevalence between children living in fluoridated and nonfluoridated communities.18
Important secular changes have occurred in the prevalence of dental caries in the United States and elsewhere. Figure 8-2 shows findings drawn from four national surveys.13,19 Two important trends can be seen in this figure. The first is that the prevalence of caries in the United States declined substantially between the early 1970s and late 1980s. Whereas the mean DMFS for U.S. children ages 5 through 17 was 7.1 during the early 1970s, this value had dropped to 2.5 by the late 1980s, a 65% reduction. At the same time, it can be seen from the figure that the proportion of DMFS that is either untreated caries or missing surfaces has also dramatically fallen during this period. As mean DMFS has fallen, the proportion of caries-free children has increased. For example, the percentage of 12- to 17-year-old children in the United States who are caries free had increased threefold from 10.4% in the early 1970s to 32.7% in the late 1980s.13,19 A similar decline in the prevalence of caries in the permanent dentition of children has occurred throughout other Western countries as well,20–23 with this decline in Western countries being widely attributed to the use of fluorides.22
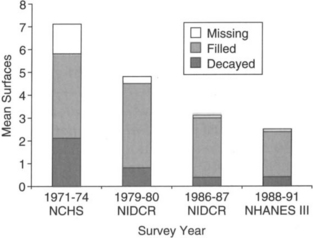
Fig. 8-2 Changes in DMFS prevalence among U.S. children, 1971-1 991.
(Data from Brunelle JA, Carlos JP: Changes in the prevalence of dental caries in U.S. schoolchildren, 1961-1 980,) Dent Res 61:1346, 1982; US Department of Health and Human Ser-vices: Oral health of United States children: the Na-tional Survey of Dental Caries in US. School Children: 7986-7987, National Institutes of Health pub no 89-2247, Washington, DC, 1989, US Government Printing Office; Kaste LM et al: Coronal caries in the primary and permanent dentition of children and adolescents 1-1 7 years of age: United States, 1988-1991,) Dent Res 75 (spec iss): 631, 1996.)
Although these findings represent significant success on the part of efforts to reduce the prevalence of caries, it is important to remember that these values represent averages over a large (5 to 17 years) age span. For example, although the mean DMFS for 10-year-old children in the 1986–1987 NIDCR national survey was 1.7, the mean DMFS was four times as high (i.e., 8.0 DMF surfaces) among 17-year-olds.10 Similarly, whereas 97% of 5-year-olds were caries free, only 16% of 17-year-olds were caries free.10 There has also been a change in the contribution of specific surface types to the total DMFS. Although the proportion of DMF surfaces that were proximal smooth surfaces has decreased by half from 24% to 12% during the past 20 years, the portion of the DMFS that is occlusal and buccal lingual surfaces has proportionally increased.10 Thus the greatest relative reductions in caries have occurred on the smooth surfaces, again strongly suggesting the role of fluoride in the decline in caries prevalence.22,24
A decline in caries prevalence has also occurred in the primary dentition.10,13,19 A comparison of the 1979—1980 and 1986—1987 NIDCR national surveys showed that mean dfs among 5- to 9-year-old children declined from 5.3 surfaces to 3.9 surfaces, a 26% decline.10 The NHANES III 1988—1991 survey reported that 50% of 5- to 9-year-old children and 83% of 2- to 4-year-old children were caries free in the primary dentition.13 A similar decline in the prevalence of caries in the primary dentition has occurred in other Western countries.20,22,23
Early Childhood Caries
A distinct form of caries in the primary dentition is early childhood caries (ECC), known formerly in the literature as “nursing caries.”25 A recent, thorough review of ECC illustrates the difficulty in describing this condition epidemiologically because of differences in clinical diagnostic criteria and case definition, study designs, and populations studied.26 For example, the review reported that the prevalence of ECC ranged from 0.8% to 64% across the 71 population studies they reviewed.26 Nevertheless, the current best estimate of ECC prevalence in the United States is approximately 5% nationwide.25,27 However, the literature further indicates important ECC prevalence difference across children of different race, ethnic, and socioeconomic background, with ethnic minority and lower socioeconomic status children being at greatest risk.28 For example, a recent report of a statewide study of Arizona preschool children who participated in federal assistance programs reported a caries prevalence of 25% by age 3 years.29 The exact etiologic mechanism(s) responsible for the development of ECC remain to be clearly defined.30,31 A recent national workshop recommended that ECC be defined as the presence of one or more primary tooth surfaces affected by caries (i.e., decayed, missing, or filled).32 This workshop further recommended that the term severe early childhood caries (S-ECC) be applied to the occurrence of caries that was either atypical, progressive, acute, or rampant in nature, using specific suggested criteria.32 The development and acceptance of consistent ECC case definitions will be an important step in clarifying both the prevalence and etiologic mechanism(s) of this disease. Despite these current limitations, preventive strategies have been proposed, based on an understanding of the set of risk and preventive factors that may play a role, including early mutans streptococci colonization and poor oral hygiene, frequent ingestion of fermentable carbohydrates, enamel hypoplasia, and limited exposure to fluorides.26,30,31,33,34 These strategies include community interventions, such as water fluoridation; professional interventions, such as early detection and sealants; and home care, including proper diet and oral hygiene habits.26
Coronal Caries in Adults
Nearly all dentate U.S. adults have at least one decayed or filled tooth.11,35 Mean DFS continues to rise with age until around 50, after which it plateaus at approximately 30 DF surfaces, as illustrated in Fig. 8-1.11,35 Data from U.S. national surveys of adults indicate that Caucasians have a significantly higher coronal DFS as compared with non-Caucasians. For example, the NHANES III survey reported that Caucasians had a mean coronal DFS twice as high as African-Americans (i.e., 24 surfaces and 12 surfaces, respectively).35 However, although surveys have estimated that the proportion of untreated coronal caries for the entire U.S. population is less than 15%, this proportion is approximately three times higher in African-American adults than in Caucasians.11,35
The prevalence of coronal caries has declined in recent decades among U.S. adults under age 45 years.36 Actual prevalence estimates vary widely, but a decrease in the prevalence of coronal caries among adults has also been observed in other Western countries.20,21,37,38
Root Surface Caries
Wide variation in the methods used to diagnose and report root surface caries, in particular in the case definition used, has made the comparison of findings from different investigations challenging.39–42 This has led to estimates of root caries prevalence that vary widely.41 The two most recent U.S. national adult surveys suggest that between 20% and 25% of U.S. adults have at least one root surface that has been affected by caries.11,35 This prevalence is virtually identical between Caucasians and African-Americans, as is the average root surface DFS, which is approximately equal to one surface.11,23 The prevalence of root caries increases with age.11,35,41,43 For example, the findings of the most recent national survey show the prevalence of root surface caries rising steadily from 7% among 18- to 24-year-olds to 56% among those 75 years and older.35 In contrast to coronal caries, the proportion of untreated root surface caries can be high (50% in the most recent U.S. national survey).35 As is the case with coronal caries, minorities have a much higher proportion of untreated root surfaces as compared with Caucasians.35 Importantly, as with coronal caries, the prevalence and incidence of root caries are lower in areas served by fluoridated drinking water.41
In the absence of pathology, root surfaces are not exposed to the oral environment and are therefore not at risk of carious attack. Thus, although root surface DFS is a useful prevalence measure, root caries incidence is best measured in terms of root surfaces at risk.42,44–46 The Root Caries Index, which counts only exposed root surfaces as being at risk for root surface caries, was developed to address this important issue.44,45
Caries “Polarization” and Risk Assessment
Descriptive caries data indicate that in addition to there having been a marked decrease in the prevalence of caries, there has also been a change in the pattern of dental caries in the United States and other countries, with most of the caries occurring among 20% to 30% of the population.13,23,47,48 For example, the NHANES III survey found that 80% of the total DMFT for children ages 5 through 17 years occurred among 25% of the children.13 These findings have led to attempts in recent years to develop methods and models by which people at high risk for future caries attack can be identified out of a population generally at low risk.49–58
The concept that caries is a multifactorial disease has long been understood and accepted, and individual factors affecting the risk for caries have been identified for many years.59 These factors include the presence of specific microorganisms, such as Streptococcus mutans; dietary factors, such as the proportion and frequency of dietary carbohydrate; salivary factors, such as salivary flow rate; and host factors, such as age and health behaviors.49,60 A series of analytic epidemiologic investigations has been conducted in an effort to identify comprehensive models of future coronal caries risk; however, the complex, multifactorial nature of the caries process makes this a challenging task.49–58 To date, past caries experience (i.e., DFS) and salivary S. mutans and Lactobacillus levels have been found to be the best predictors of future coronal caries risk.50–5861 Studies have also identified sugar consumption as an indicator of future caries risk.62,63 Studies indicate that factors such as fluoridation status, past root caries experience, age, number of teeth, gingival recession, number of decayed coronal surfaces, and use of sugared foods are predictors of root surface caries.41,63,64
EPIDEMIOLOGY OF PERIODONTAL DISEASE
Periodontal disease has been defined by Loe65 as “a group of lesions affecting the tissues surrounding and supporting the teeth in their sockets.” The vast majority of periodontal disease cases can be classified as either gingivitis or periodontitis.
Gingivitis is a disease characterized by inflammation restricted to the gingival soft tissues, with no loss of alveolar bone or apical migration of the periodontal ligament along the root surface.66 Clinically, the signs of gingivitis are erythema, edema, and gingival bleeding.66 Gingival diseases can be divided into two main categories: those that are associated with the presence of dental plaque and those that are not.67 Plaque-induced gingival disease can be further categorized as to whether it is related to the presence of plaque only, or whether it is also associated with systemic conditions, such as the presence of blood dyscrasias; use of medications, such as oral contraceptives; or malnutrition, especially ascorbic acid (vitamin C) deficiency.68 Periodontitis is characterized by inflammation that extends beyond the gingiva to the periodontal structures, causing destruction of the periodontal ligament attachment and alveolar bony support and leading to migration of the junctional epithelium apical to the cementoenamel junction.69,70 The two major categories of periodontitis are chronic periodontitis (formerly classified as adult periodontitis) and aggressive periodontitis (formerly characterized as early-onset or juvenile periodontitis).67 These changes in terminology reflect the consensus of a recent workshop organized by the Academy of Periodontology.71,72 The decision to abandon the terms adult periodontitis and early-onset or juvenile periodontitis was based on the observation that these forms of periodontitis can each occur across a wide age range, affecting children, adolescents, and adults.72 In addition to these two major categories, other categories of periodontitis include periodontitis as a manifestation of systemic disease, such as hematologic or genetic disorders; necrotizing periodontal diseases, a condition usually seen in the presence of underlying systemic conditions such as human immunodeficiency virus (HIV) infection; abscesses of the periodontium, a condition that, although associated with other forms of periodontitis, is considered a distinct condition in terms of diagnosis and treatment; periodontitis associated with endodontic lesions; and periodontitis associated with developmental or acquired conditions such as tooth anatomy or the presence of dental restorations.67 Clinically, the signs of periodontitis can include loss of soft tissue attachment, gingival recession, tooth migration, mobility, and tooth loss.66,73,74 Most cases of gingivitis and periodontitis occur as a result of the presence of bacterial plaque on the gingiva and subgingival tooth surfaces and calculus.69 It is generally accepted that periodontal disease begins as gingivitis, which progresses, only in some individuals, to periodontitis, with severe periodontitis affecting only a small percentage of the population.66,75
Measurement of Periodontal Disease
Historically, several indices were developed in an attempt to provide a standardized method of measuring periodontal disease among groups of people in epidemiologic studies, most notably the Periodontal Index and the Periodontal Disease Index.76,77 Both of these indices have been criticized on methodologic grounds, especially because they combine gingivitis and periodontitis measures into a common score.78,79 For this reason, neither of these indices is considered the best method to measure periodontal disease. Another index, which gained considerable international popularity, is the Community Periodontal Index of Treatment Needs (CPITN), developed by the World Health Organization (WHO) to provide a means to summarize treatment needs.80,81 To do this, the CPITN combines an assessment of gingival health, pocket depth, and the presence of supragingival and subgingival calculus.80,81 Proponents of the CPITN state that the CPITN allows for a rapid, simple, uniform method by which the average periodontal status and treatment needs of international populations can be determined using minimal equipment. It has been further argued that the use of a widely understood index facilitates the formulation of international goals for periodontal health and that this index has been used in more than 100 countries to generate data for the WHO Global Oral Data Bank.82 However, critics of the CPITN argue that the combination of gingival health, pocket depth, and presence of calculus into a combined score is not consistent with current approaches to describing periodontal disease and that failure of the CPITN to measure gingival recession leads to an inaccurate estimate of attachment loss.78 A consensus report of the American Academy of Periodontology concluded that it is an inappropriate measure with which to assess the prevalence and severity of periodontal disease.83,84
The Gingival Index (GI) was introduced to provide an index that measured only inflammation of the gingiva and that would allow a clear distinction to be made between the location or quantity of gingivitis and the severity or quality of the gingivitis.85,86 The GI accomplishes this by applying a four-category qualitative assessment (normal, mild, moderate, or severe inflammation) to four sites on each examined tooth. These values can then be averaged to yield a score for the individual.85,86 The GI has become a mainstay index of gingivitis.87 At the same time, it has become generally accepted that periodontitis should be measured and reported separately from gingivitis, characterized in terms of extent, or number of affected sites, and severity in terms of loss of periodontal attachment measured in millimeters.72
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses



