CHAPTER 8
Augmentation Grafting of the Maxillary Sinus for Placement of Dental Implants
Dental implant placement in patients who are edentulous in the posterior maxilla can be difficult for a variety of reasons, including increased pneumatization of the maxillary sinus (and thus close approximation of the maxillary sinus floor to the alveolar crestal bone) or inadequate ridge width.1 Sinus pneumatization, which typically occurs with aging, often minimizes or completely eliminates the vertical bone available for endosteal implant placement in the maxillary sinus. Often, the bone partition between the alveolar mucosa and the maxillary sinus is as thin as 1 mm (Fig 8-1).2
Since the mid-1990s, bone grafting the sinus floor to increase vertical height and improve bone quality for implant placement has become increasingly successful. It is an excellent and predictable procedure for treating patients who have a severely atrophic posterior maxilla.3 Using a pull-out test of dental implants in various types of bone, one study found that bone-grafted areas tend to have an even higher bone-to-implant contact and greater pull-out resistance than normal bone. Thus, bone grafting around implants is recommended in sites that are deficient in bone volume or density or in sites such as the maxilla that have a history of implant failure.4
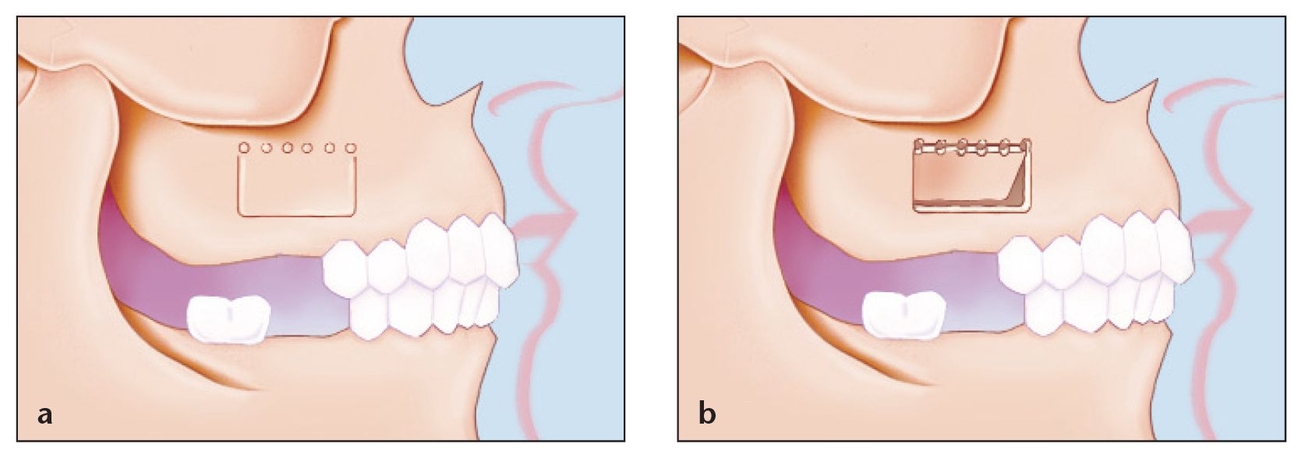
Grafting of the antral floor was originally developed and described by Tatum in the early 1970s (Fig 8-2).5, 6, 7 Initially, he used an alveolar crestal access to the maxillary sinus. Subsequently, a modified Caldwell-Luc procedure was developed in which he approached the sinus by infracturing the lateral wall of the maxilla and using the wall to elevate the maxillary sinus membrane (Fig 8-3). An autogenous bone graft was then placed in the area previously occupied by the inferior third of the sinus. This technique provided adequate bone in the posterior maxilla, permitting various implant placement options.
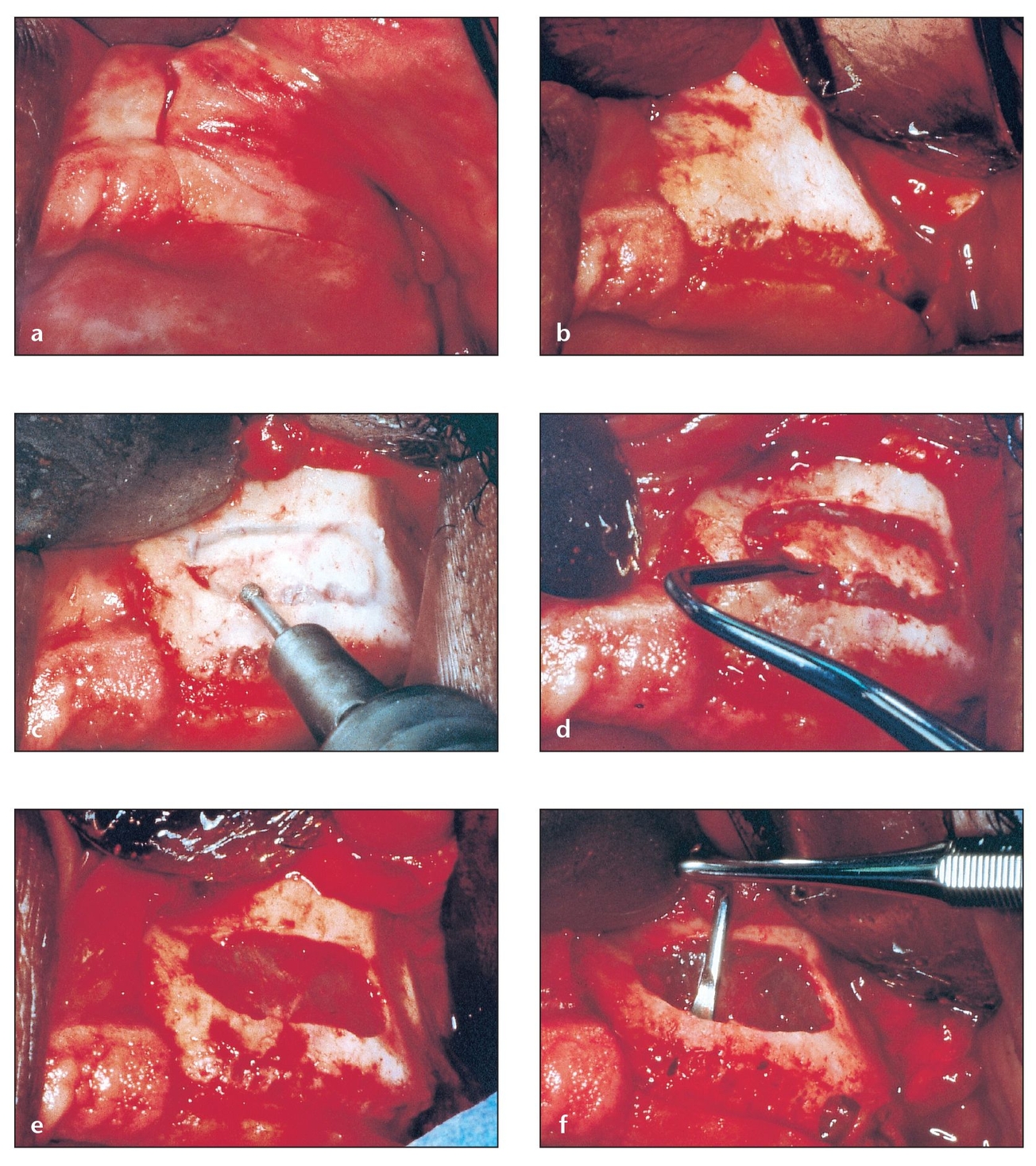
Fig 8-1 Sinus pneumatization often minimizes or eliminates the vertical bone available for implant placement in the maxillary sinus, necessitating bone grafting of the sinus floor to increase vertical height and improve bone quality.
(a) Maxillary ridges showing sufficient width for implant placement but inadequate height because of sinus cavity hyperpneumatiza-tion. The maxillary sinus lateral wall and floor are approximately 1 to 2 mm thick. An incision is made with a sharp no. 15 blade down to the bone, resulting in a clean cut through the tissues.
(b) A mucoperiosteal flap is reflected with a sharp periosteal elevator to avoid shredding of mucosa or periosteum. Vertical incisions permit adequate elevation of this flap for good visibility of the lateral wall of the maxilla.
(c) The osteotomy is shaped according to the sinus cavity. This step should be performed carefully, as the thickness of bone varies greatly from one patient to another.
(d) The island of bone that remains after the osteotomy is carefully elevated. The resistance of residual bone is variable, so this should be done slowly to prevent or minimize tears. Note the minimal thickness of the bone.
(e) The maxillary sinus membrane is exposed and ready to be reflected. Smooth edges facilitate the next step.
(f) The sinus membrane is reflected with specially designed curettes. Note that there are areas of the residual alveolar crest that are 0 mm thick.
(a) The classic window design for augmentation grafting of the maxillary sinus. A rectangular or trapezoidal osteotomy is created and the superior portion is not contiguous. The underlying schneiderian membrane is left intact. A modified and recommended technique is presented later in this chapter.
(b) The bony island is fractured with an osteotome and mallet and elevated superiorly while simultaneously elevating the underlying schneiderian membrane.
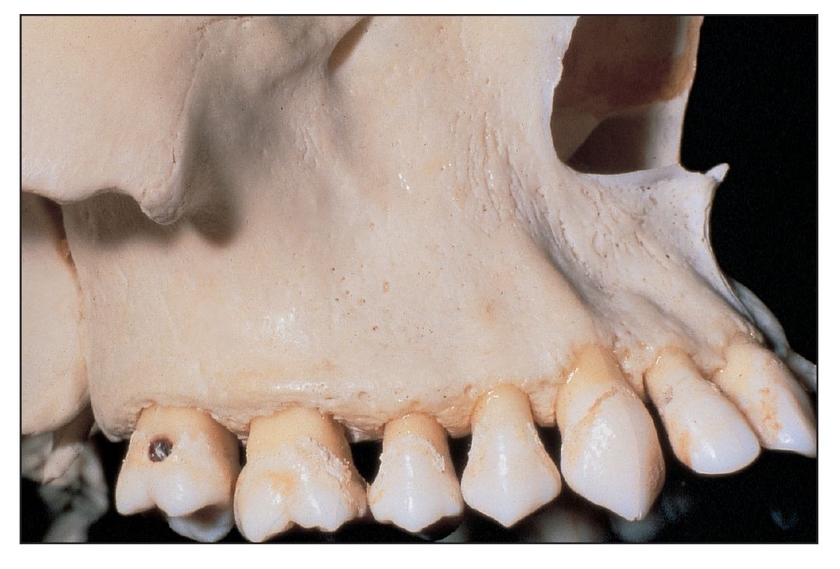
Lateral wall of the maxilla. Note the relationship of the infraorbital foramen and the sinus area. Also note the position of the zygoma in relation to the area for the osteotomy; this will be a landmark to use when designing the osteotomy. It is important to maintain the window inferior to the zygoma to minimize the chances of damaging the infraorbital nerve with the bur or the retractor.
In 1980, Boyne and James described a similar clinical procedure and demonstrated bone formation in the maxillary antrum following placement of autogenous marrow and cancellous bone in the maxillary sinus.8 In 1984, Misch modified the technique, combining sinus augmentation and blade-vent implant placement in the same procedure.9 In 1997, a further modified technique was published by Garg and Quinones in which sinus augmentation and rough-surface implants were combined and the window shape and design were modified along with recommended instrumentation 10 (Fig 8-4). These procedures differ in the initial surgical approach, the type or donor site of grafting material, and the type of implant used.
Sinus lift grafting and implant placement can be accomplished as either a one-or two-step procedure. Many authors have reported good initial results with both approaches. 1, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 If there is sufficient alveolar bone width and only partial pneumatization of the sinus, bone grafting and implant placement can be performed at the same time. This one-step approach offers the advantages of minimizing total treatment time by eliminating a second surgical procedure and of allowing a coordinated consolidation of the graft around the implant1 (Fig 8-5).
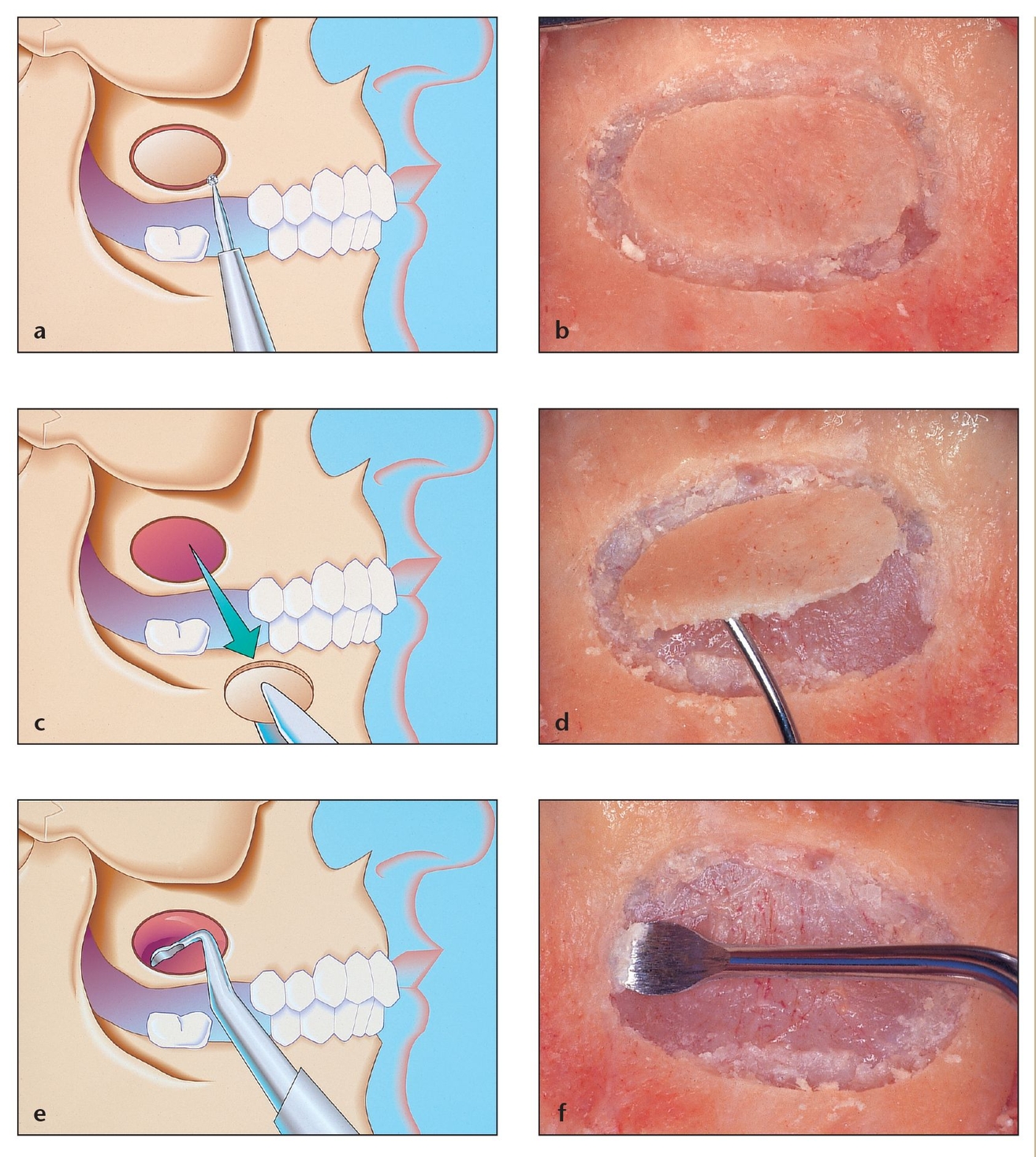
Fig 8-4 Sinus augmentation grafting technique using rough-surface implants and a modified window shape and design.
(a) The ideal shape of the osteotomy should be ovoid and contiguous. In this manner, the chances of schneiderian membrane perforation with sharp corners from a rectangular or trapezoidal osteotomy are minimized. This also minimizes membrane perforations due to sharp edges arising from a greenstick-fractured area superiorly.
(b) Osteotomy perfomed on a cadaver specimen. The size and shape of the osteotomy should follow the contours of the maxillary sinus.
(c) Removal of the island of bone. This should be performed gently to minimize the chances of perforating the underlying membrane.
(d) Elevation of the island of bone in a cadaver specimen.
(e) Sinus membrane elevation using a specially designed curette. Sharp curettes should be used and the membrane should be reflected off the bone as opposed to attempting to simply push it off the bone.
(f) Sinus membrane elevation with a curette in a cadaver specimen.
(g) Coronal view of sinus membrane elevation. Note that the inferior portion of the window is approximately 3 mm above the sinus floor. This allows the surgeon to avoid some 1 to 2 mm sinus septa during elevation of the sinus window and allows for a small lip of bone to help contain the graft material. The superior portion of the window depends on the size of the implant that will be placed. The superior portion of the window should be measured from the ridge crest and should be at least the same height as the implant that is planned for the area.
(h) Membrane elevation in a cadaver specimen.
(i) Sinus cavity grafted with the amount of material needed for future implant placement. Note that this does not obliterate the entire sinus cavity.
(j) Grafting the sinus cavity returns the original contour of the lateral wall of the maxilla on this cadaver specimen.
(k) Coronal view of the grafted sinus cavity after some maturation of the graft has occurred.
(l) Coronal view of the implant site after the implant is placed and surrounded by sufficient bone.
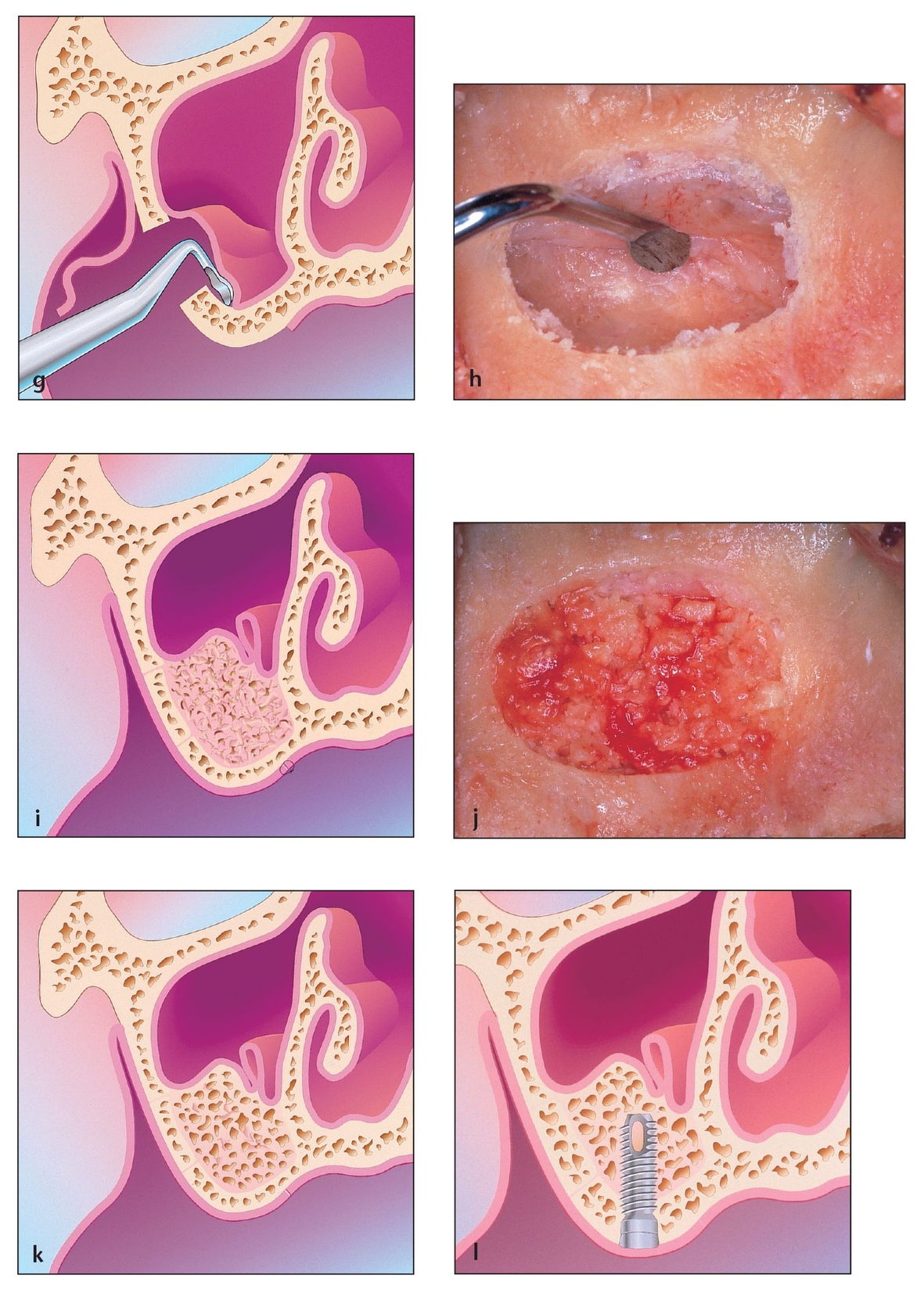
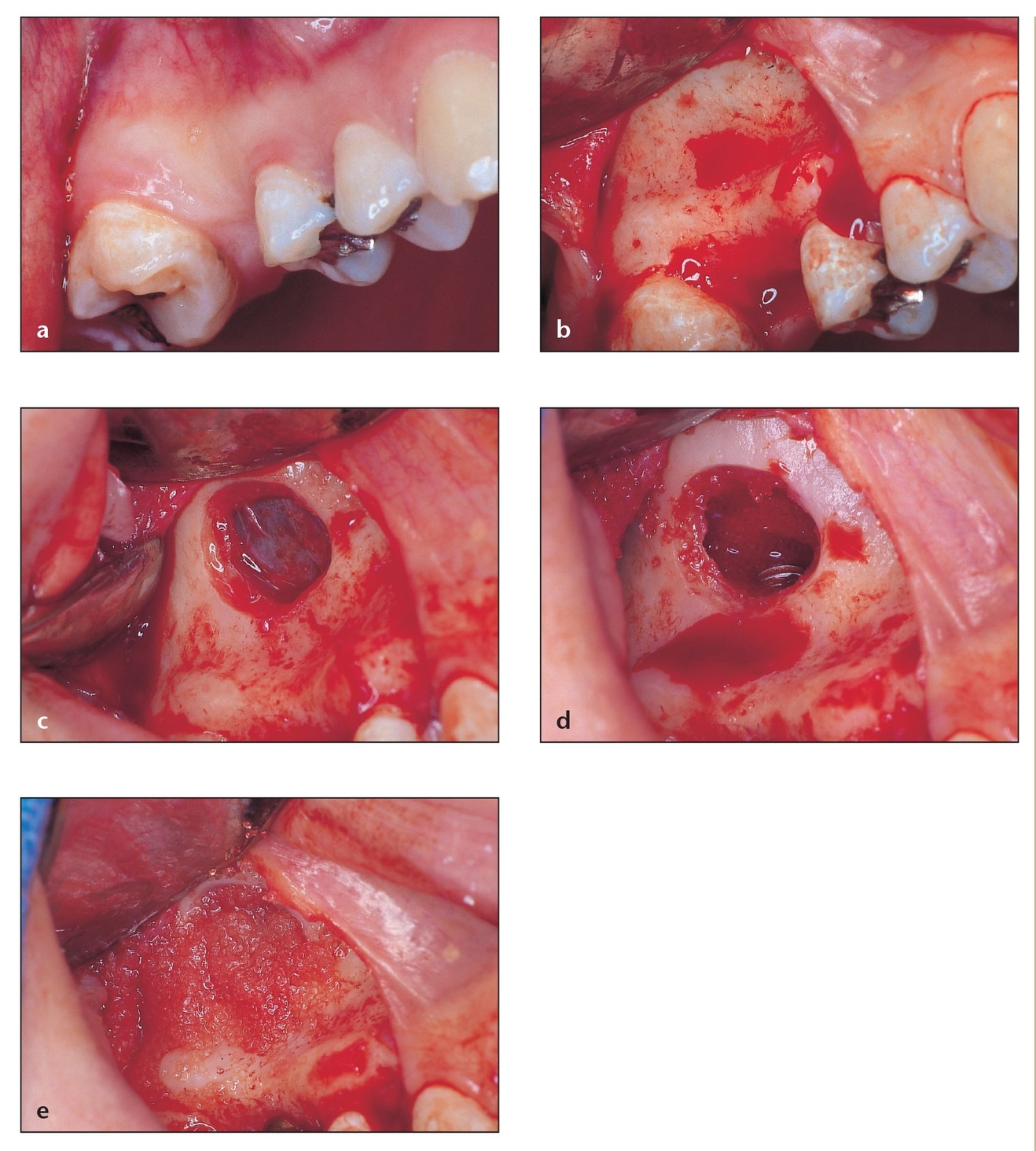
Fig 8-5 If there is sufficient alveolar bone width and only partial pneumatization of the sinus, bone grafting and implant placement can be performed at the same time.
(a) Sites with just one missing tooth can have enough pneumatization that the area requires sinus grafting. Bone grafting and placement can be performed simultaneously. The vertical release should be extended to the depth of the vestibule.
(b) The size of the flap is determined according to the site, but achieving good visualization always should be a consideration.
(c) In this case, a small osteotomy can be performed, thus avoiding any damage to adjacent teeth. Smooth edges are recommended to facilitate reflection.
(d) Integrity of the sinus membrane can be assessed by asking the patient to take a few deep breaths through the nose. At this point the implant osteotomy is performed, the medial half of the sinus is grafted, and the implant is placed.
(e) The sinus is then filled flush with the existing contours. A resorbable membrane extending beyond the borders of the window 3 mm circumferentially will be placed and the flap closed primarily.
In the past, available host bone measuring less than 5 mm in height was deemed inadequate to mechanically maintain an endosteal implant. Thus, in these cases simultaneous bone grafting and implant placement were contraindicated in favor of the two-step approach, in which implant placement is delayed until 4 to 6 months after graft placement.12,13 In recent years, this concept has been challenged, with success reported using the one-step approach for posterior maxillary ridges measuring as little as 1 mm in height.2, 26, 27, 28, 29 The critical factor appears to be adequate ridge width for placement of the intended implant (Fig 8-6).
Fig 8-6 Recent studies show high success rates following simultaneous implant placement with sinus grafting, even in cases with as little as 1 mm of crestal bone height.
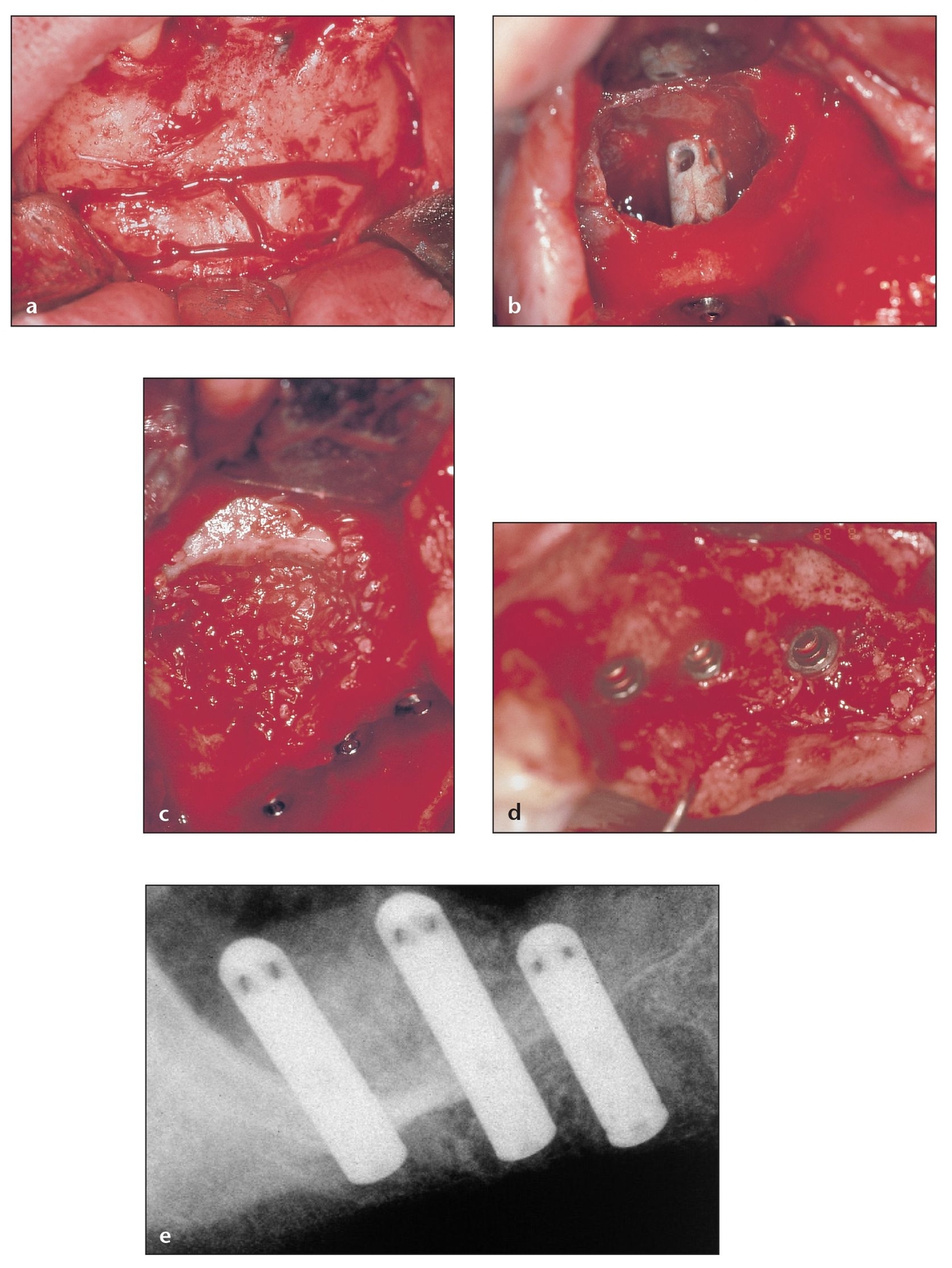
(a) Harvest of autogenous bone from anterior mandible to use for the graft. When performing grafting for hyperpneumatized sinuses, use of autogenous bone is recommended.
(b) Simultaneous implant placement with sinus grafting in cases with as little as 1 mm of crestal bone height must be performed by an experienced clinician, and a minimum of 8 mm of bone width is required. The lateral aspect will then be grafted.
(c) Multiple implants were placed simultaneously in the graft with good initial stabilization of the implants in spite of the minimal residual height of bone. Packing the bone in a careful, meticulous manner—densely in the sinus and around the implants—ensures that the implants remain in the same position.
(d) After 5 months of maturation, excellent results can be seen without any complications for stage 2 surgery.
(e) Radiographically, the bone is still mineralizing, and the position of the implants has been maintained as expected.
Because few vital anatomic structures encroach upon the surgical site, the risks with sinus lift grafting are negligible, morbidity is low, and postoperative complications can be treated relatively easily with medical or surgical intervention. Bone re-sponse is excellent, and different graft materials produce bone that is demonstrable on histologic examination.26 The graft and new bone appear to remodel in response to functional loading. The prosthetic alternatives are also predictable; fixed, fixed removable, or removable prosthetic reconstructions can be placed over implants within the sinus graft.1
Maxillary Sinus Anatomy
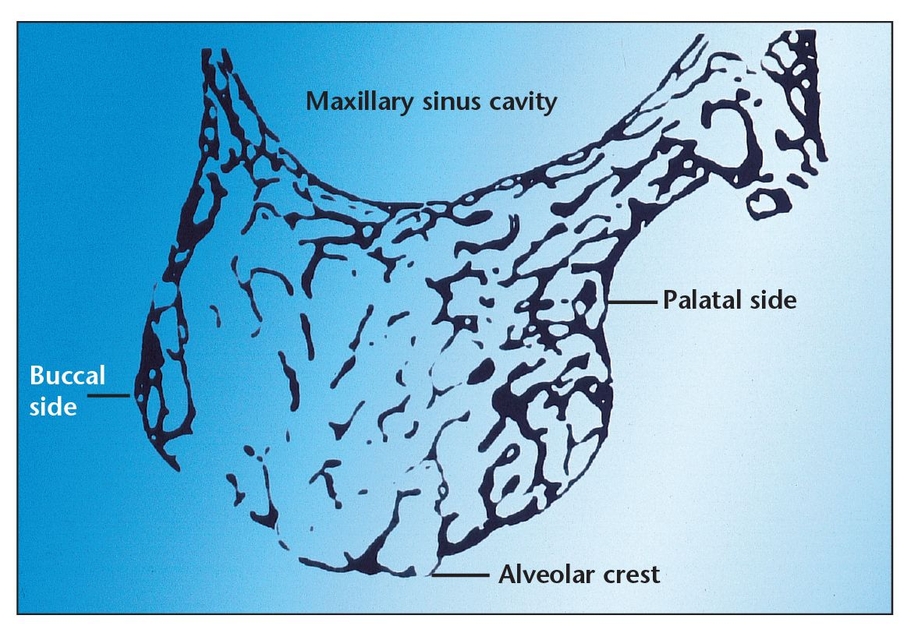
Maxillary bone is primarily medullary (ie, spongy) (Fig 8-7) and finely trabecular. The quantity and osseous density of bone in this area is lower than that of premaxillary or mandibular bone. The adjacent cortices consist of compact bone; however, they are generally very thin, providing minimal strength compared with the cortices surrounding the mandible. Because of its spongy nature, medullary bone must establish a stress-bearing surface next to an endosteal implant in order for the functioning implant to remain stable and be able to transmit physiologic load to the supporting bone.5, 30
The maxillary sinus is an approximately 15-mL-volume air space, although the actual size depends on the amount of resorption that has occurred. It resembles a sloped paperweight, with its largest and only flat side composing the medial wall (which is also the lateral wall of the nasal cavity).31, 32, 33 Septa may divide the sinus into two or more cavities that may communicate. The sinus begins to form in the second to third year of life, and its formation is nearly complete by 8 years of age. It has a nonphysiologic drainage port high on the medial wall (maxillary ostium) that drains into the middle meatus of the nose. The ostium is considered nonphysiologic because it serves as an overflow drain rather than as a dependent complete drainage system.
The bony walls of the sinus are thin, except for the anterior wall and the alveolar ridge in the dentate individual. In the edentulous person, the alveolar bone is frequently atrophied and may be only 1 to 2 mm thick, making it unsuitable as an implant site without appropriate grafting. Thus, the purpose of sinus lift surgery is to restore a sufficient amount of alveolar bone so that implants can be successfully placed.
The maxillary sinus is lined with a pseu-dostratified columnar epithelium, which is also called the schneiderian membrane. Beneath the surface epithelium is a loosely cellular but highly vascular thin tissue. Beneath this, in all areas, is a periosteum. The delicate mucosa of the sinus attaches to the periosteum on its osseous surface. However, this feature is not an important source of bone formation in sinus lift surgery. A thin layer of respiratory epithelium, which lines the schneiderian membrane, cannot be differentiated from the periosteum of the bones to which it is firmly affixed.
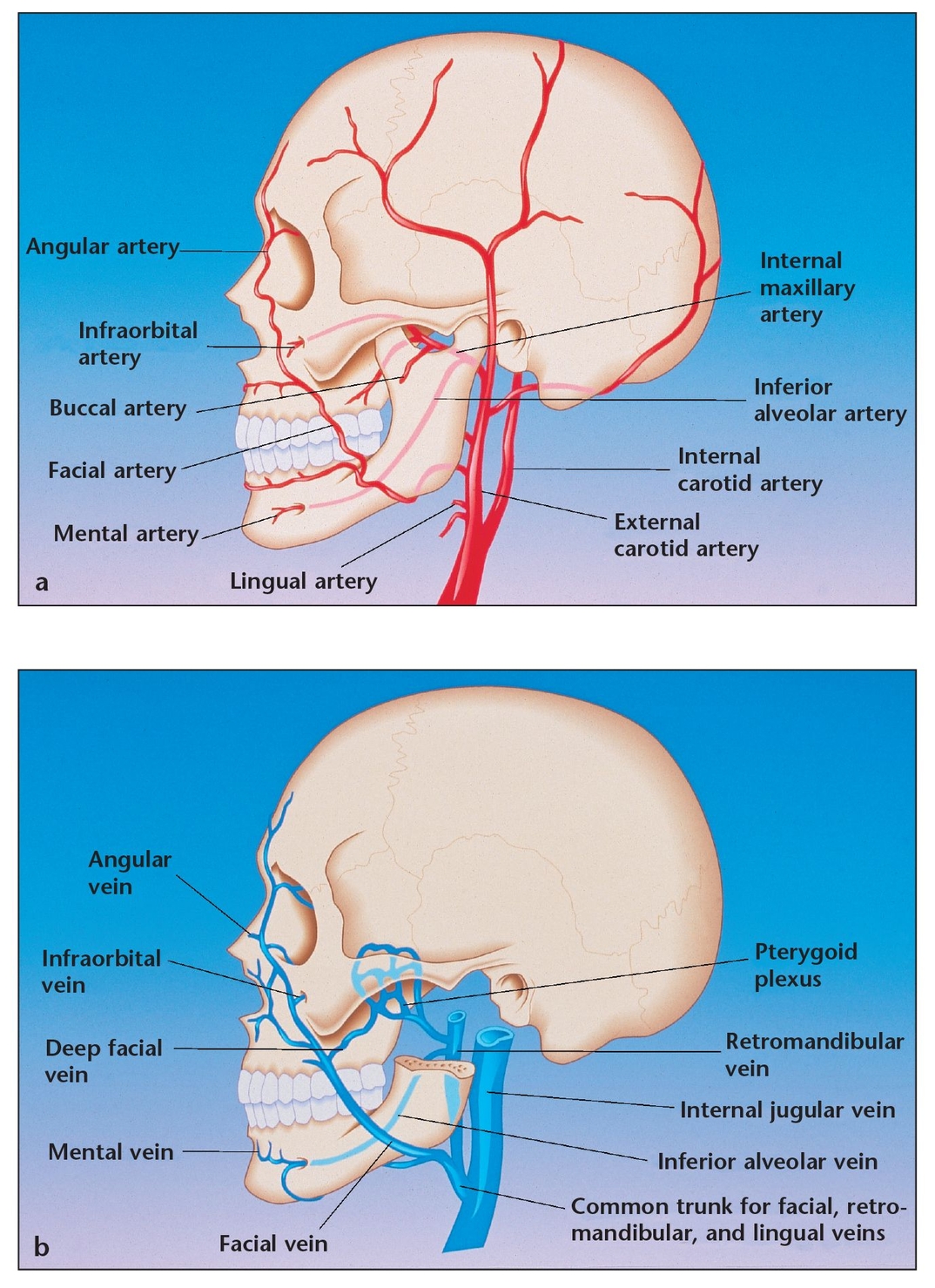
The blood supply to the maxilla normally emanates from three parent arteries—the superior labial, anterior ethmoidal, and, primarily, the internal maxillary arteries. The area of sinus lift surgery is mainly supplied by branches from the internal maxillary artery. The sinus floor derives some of its blood flow from the greater and lesser palatine vessels as well as the incisal artery, a terminal branch of the sphenopalatine artery (which is yet another portion of the internal maxillary artery). These vessels penetrate the bony palate and ramify within the sinus floor and its medial and lateral walls. Another vascular contributor is the posterosuperior alveolar artery, which enters the maxilla in the superior tuberosity area to supply most of the posterior and lateral walls. The infraorbital branch of the internal maxillary artery helps to supply blood to the supero-lateral sinus area. The anterior ethmoidal artery, which is a terminal branch of the internal carotid system (via the ophthalmic artery), supplies the superomedial sinus area (Fig 8-8).
Coronal aspect of a decalcified histologic section of a maxillary ridge and its relationship to the sinus cavity. Note the spongy nature of the bone in this area.
(a) Blood supply to the sinus starting at the common carotid artery.
(b) Venous drainage from the facial area.
Mechanisms of Bone Grafting
Transplanted osteogenesis is another term for bone grafting; the phrase emphasizes that bone is dynamic and forms by cellular regeneration, which produces osteoid that becomes mineralized. A graft is not a solid bone block that heals into place.34 Bone grafting is accomplished through osteogenesis, osteoinduction, and/or osteoconduction. 35, 36, 37, 38 Osteogenesis refers to the formation and development of bone by osteocompetent cells. Osteogenic graft material, which is derived from or composed of tissue involved in the natural growth and repair of bone, can encourage bone formation in soft tissues and can stimulate faster bone growth in bone implant sites. Osteoinduction is the process of activating osteogenesis by recruiting cells from the surrounding natural bone that then differentiate into bone-forming cells. Osteoinductive grafts can enhance bone regeneration, sometimes even resulting in the extension or growth of bone where it is not normally found. Osteoconduction is the process by which the graft material acts as a nonviable scaffold onto and within which the patient’s own natural bone grows. Osteoconductive grafts are conducive to bone growth and allow apposition from existing bone, but they do not produce or trigger bone formation themselves when placed in soft tissue.
Bone-Grafting Materials
Many materials have been used for sinus lift procedures, including autogenous bone,11–16 bone allografts,17, 35, 38, 39, 40, 41, 42, 43 and alloplasts such as tricalcium phosphate (TCP), resorbable and nonresorbable hydroxyapatite, 1, 38, 44, 45, 46 bovine-derived bone mineral, 47 and bioactive glasses. An ideal graft is nontoxic, nonantigenic, noncarcinogenic, strong, resilient, easily fabricated, able to permit tissue attachment, resistant to infection, readily available, and inexpensive.48
Autogenous Bone
To date, there is no official consensus as to which graft material or combination of materials is best for augmenting the sinus antral void created by the sinus lift operation. 1, 49, 50, 51 Autogenous bone has long been considered the gold standard among grafting materials because of its highly osteogenic, osteoinductive, and osteoconductive properties, a combination not found in the alternatives.52 These properties allow bone to form more rapidly and in conditions where significant bone augmentation or repair is required. In a 1993 histomorphometric study of patients who underwent maxillary sinus augmentation, Moy et al assessed the bone composition of four different graft materials using biopsies taken from graft sites at the time of implant placement.49 Particulated autogenous chin grafts contained 59.4% bone; composite grafts of hydroxyapatite and chin bone contained 44.4% bone; grafts of hydroxyapatite alone contained 20.3% bone; and grafts of demineralized freeze-dried bone alone contained 4.6% bone. Lorenzetti et al performed a similar study, which revealed that autogenous chin grafts contained 66% bone; autogenous iliac bone grafts contained 53% bone; and 50-50 composite grafts of autogenous chin bone and hydroxyapatite granules contained 44% bone.53
Cancellous particulated bone from the iliac crest continues to be an excellent source of autogenous graft material,54 as is that from the tibial plateau (the procedure is described in greater detail in chapter 7). Intraoral sites such as the mandibular symphysis, maxillary tuberosity, ramus, and exostoses and debris from an implant osteotomy have also been used with success.13, 17, 18, 38, 55 Mandibular bone grafts reportedly resorb less than do iliac crest grafts,13,18 and the procedure can be easily accomplished in an office setting with the patient under parenteral sedation and local anesthesia (the procedure is described in greater detail in chapters 2, 5, and 6). Thus, there is no postoperative hospitalization, which results in lower costs and better patient acceptance.
A disadvantage of intraorally obtained bone grafts is that donor sites provide a smaller volume of bone than what can be obtained from the iliac crest or tibial plateau. A typical sinus requires approximately 4 to 5 mL of bone volume for grafting for dental implants. The total graft volume required is naturally dependent on the amount of bone resorption (sinus pneumatization and ridge resorption) that has occurred at the time the patient presents for surgery. Typically, 5 mL of bone can be harvested from the anterior mandible, 5 to 10 mL from the ascending ramus, 20 to 40 mL from the tibial head, 70 mL from the anterior ilium, and approximately 140 mL from the posterior iliac crest.
The use of cortical and corticocancellous blocks adapted to the sinus floor has also been reported, although healing time is longer compared with that associated with particulated graft material.56 In a 6-year follow-up investigation of 216 sinus lift procedures with immediate placement of 467 implants into bone measuring 1 to 5 mm in height, Khoury observed the best bone regeneration in patients grafted completely with autogenous material comprising a percentage of cortical bone.2
The choice of donor site usually depends on the volume and type of bone desired. In extremely healthy patients, patients with minimal sinus resorption, and patients who refuse to undergo an extraoral bone graft harvest, it maybe appropriate to expand the volume of autogenous bone harvested intraorally by combining it with other graft materials, such as allografts or alloplasts. However, some recent studies indicate that bone formed in autogenous bone–grafted sinuses is retained signficantly longer than in sites grafted with a combination of autogenous and demineralized freeze-dried bone allografts (DFDBA).57 Lorenzetti et al showed that in maxillary sinuses grafted with a combination of autogenous bone and hydroxyapatite granules, soft tissue prevailed over bone, and a year after placement the hydroxyapatite granules were clearly distinguishable and surrounded by only a very thin layer of bone.53
Allografts
Bone allografts such as freeze-dried bone allografts (FDBA) or DFDBA may be cortical or trabecular. They are obtained from cadavers or living donors other than the patient, processed under complete sterility, and stored in bone banks. Fresh allografts are the most antigenic; however, this antigenicity can be reduced considerably by freezing or freeze-drying the bone, as is customary.39
Whether these grafts form bone by osteoinduction, osteoconduction, or some combination of both is the subject of continued debate. In the 1960s, Urist suggested that allografts form bone by osteoinduction because they contain osteoinductive proteins called bone morphogenetic proteins (BMPs).58 FDBA can be used in either a mineralized or demineralized form. Both FDBA and DFDBA contain BMPs; however, in the quantities used clinically, the amount of BMPs is generally inadequate to account for osteoconduction. Demineralization removes the mineral phase and purportedly exposes the underlying bone collagen and growth factors, particularly BMPs.35,40,41 Although the demineralization process exposes growth factors, it also destroys approximately half of the growth factors contained in FDBA. Additionally, the demineralization process removes the mineral portion of the graft (hydroxyapatite), which is critical for maintaining the matrix of the grafted site and providing for osteoconduction. Several authors have since challenged this theory based on unpredictable results with DFDBA, suggesting that these allografts may contain inconsistent and often inadequate levels of BMPs because of factors such as handling and processing technique.59, 60, 61, 62 One study suggested that using DFDBA in combination with hydroxyapatite may somewhat improve its effectiveness.54 These concerns are valid; hence, the author recommends FDBA rather than DFDBA for bone grafting. This is discussed in more detail in chapter 2.
Irradiated cancellous bone has also been used as a substitute graft material for autogenous bone.42,43 However, by using mineralized FDBA, a local substrate of mineral is provided for the graft and no BMPs are destroyed in the demineralizing process. Jensen and Greer found that radiated mineralized allografts used in conjunction with maxillary antroplasty, a screw-form implant, and an expanded polytetrafluoroethylene (e-PTFE) membrane barrier provided more predictable ossification than demineralized cancellous allograft.50 They concluded that this graft material was the best option other than autogenous bone.
Advantages of allografts include ready availability, minimization of the amount of autogenous bone harvested from the patient, reduced anesthesia and surgical time, decreased blood loss, and fewer complications. 38 The disadvantages are primarily their dimished capacity to produce bone as compared to autogenous bone, and perhaps the theoretical disadvantages associated with tissues transplanted from another individual 35,38,46 (cadaver bone can be rejected like other transplanted tissues or organs). Technical problems include the precision required to insert bulk allografts, the necessity for rigid fixation to the host bone to obtain successful union, and the high rates of infection, nonunion, and graft fracture.35,39 Because allografts are not osteogenic, adding this material to autogenous bone means that bone formation will proceed more slowly and result in less volume than with purely autogenous grafts.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


