Surface Disinfection and Treatment Room Preparation
Learning Objectives
1 Pronounce, define, and spell the Key Terms.
2 Demonstrate placing and removing surface barriers.
3 Describe the levels of disinfectants and explain the indications for the use of each.
4 Name the governmental agency that is responsible for registering disinfectants and sterilants.
5 Discuss several considerations when selecting a surface disinfectant.
7 Demonstrate cleaning and disinfecting surfaces and equipment in a dental treatment room.
Key Terms
Alcohol
Antiseptic
Bioburden
Biofilm
Chlorine Dioxide
Critical Instrument
Disinfection
Glutaraldehyde
Iodophors
Noncritical Instrument
Ortho-Phthalaldehyde (OPA)
Precleaning
Semicritical Instrument
Sodium Hypochlorite
Surface Barriers
Synthetic Phenol
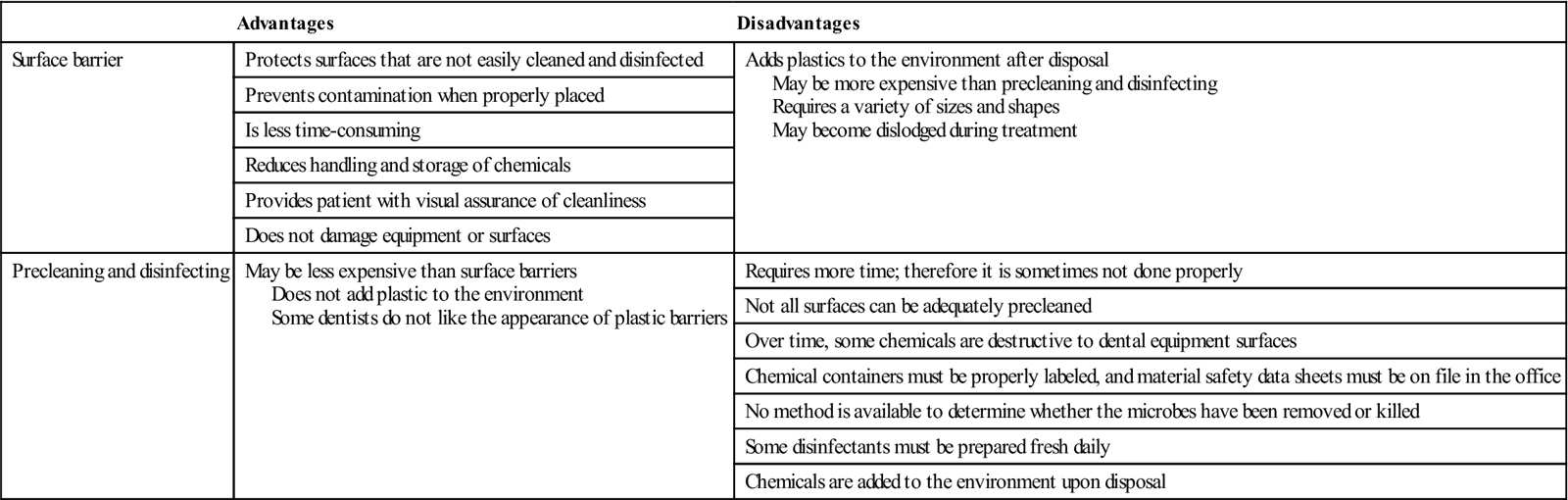
During patient treatment, the equipment and treatment room surfaces become contaminated with saliva, or by aerosol containing blood and/or saliva. A primary source of contamination occurs when a member of the dental team touches surfaces with contaminated gloves. Although no cases of cross-infection have been linked to dental treatment room surfaces, cleaning and disinfection of these surfaces are important components in an effective infection control program. In addition, the Occupational Safety and Health Administration (OSHA) Bloodborne Pathogens Standard requires that contaminated work surfaces be disinfected between patient visits. Two methods are used to deal with surface contamination. One method is to prevent the surface from becoming contaminated by using a surface barrier. The other is to preclean and disinfect the surface between patients. Advantages and disadvantages of both methods are known, and most dental offices use a combination of the two methods (Table 7-1).
TABLE 7-1
Comparison of Surface Barriers versus Precleaning and Disinfection
< ?comst?>
| Advantages | Disadvantages | |
| Surface barrier | Protects surfaces that are not easily cleaned and disinfected | Adds plastics to the environment after disposal May be more expensive than precleaning and disinfecting Requires a variety of sizes and shapes May become dislodged during treatment |
| Prevents contamination when properly placed | ||
| Is less time-consuming | ||
| Reduces handling and storage of chemicals | ||
| Provides patient with visual assurance of cleanliness | ||
| Does not damage equipment or surfaces | ||
| Precleaning and disinfecting | May be less expensive than surface barriers Does not add plastic to the environment Some dentists do not like the appearance of plastic barriers |
Requires more time; therefore it is sometimes not done properly |
| Not all surfaces can be adequately precleaned | ||
| Over time, some chemicals are destructive to dental equipment surfaces | ||
| Chemical containers must be properly labeled, and material safety data sheets must be on file in the office | ||
| No method is available to determine whether the microbes have been removed or killed | ||
| Some disinfectants must be prepared fresh daily | ||
| Chemicals are added to the environment upon disposal |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Surface Barriers
Surface barriers are used to prevent contamination on the surface, so it will not have to be cleaned and disinfected in-between patients.
Types of Surface Barriers
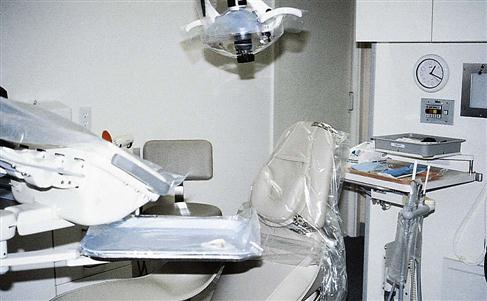
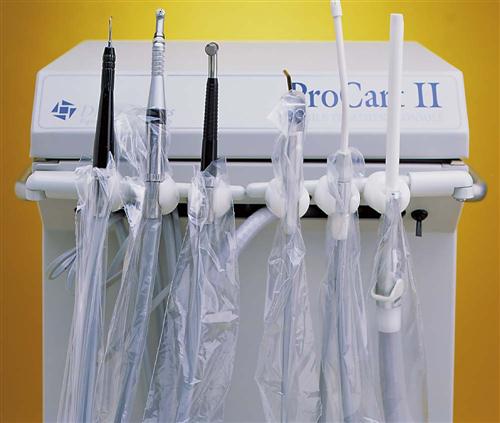
A wide variety of surface barriers are available today. All should be resistant to fluids to keep microorganisms in saliva, blood, and other liquids from soaking through to contact the surface underneath. Some plastic bags are designed especially to fit the shape of items, such as the dental chair, an air-water syringe, hoses, pens, light handles, etc. Plastic barrier sticky tape is frequently used to protect smooth electrical surfaces, such as touch pads on equipment or electrical switches on chairs or x-ray equipment. Aluminum foil can also be used because it is easily formed around any shape (Procedure 7-1 and Figures 7-1 to 7-3).
Precleaning and Disinfection
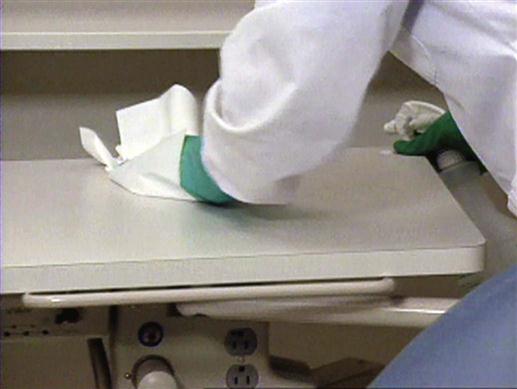
Precleaning and disinfecting techniques are most effective when used on contaminated treatment room surfaces that are smooth and easily accessible (Figure 7-4). Always wear your utility gloves, mask, protective eyewear, and protective clothing when precleaning and disinfecting (Procedure 7-2).
Precleaning
Precleaning means to clean before disinfecting. All contaminated surfaces must be precleaned before they can be disinfected. This reduces the number of microbes and removes the blood and/or saliva (also called bioburden). Not all types of disinfectants contain a precleaning agent.
Regular soap and water may be used for precleaning, but it is more efficient to select a disinfectant that contains detergents for both the precleaning step and the disinfecting step. Remember: If a surface is not clean, it cannot be disinfected.
Disinfection
Disinfection is intended to kill disease-producing microorganisms that remain on the surface after precleaning. Spores are not killed during disinfecting procedures. Do not confuse disinfection with sterilization. Sterilization is the process in which all forms of life are destroyed. Sterilization techniques are discussed in Chapter 8.
The term disinfectant is used for chemicals that are applied to inanimate surfaces (such as countertops and dental equipment), and the term antiseptic is used for antimicrobial agents that are applied to living tissue. Disinfectants and antiseptics should never be used interchangeably because tissue toxicity and damage to equipment can result.
Levels of Disinfectants
The Environmental Protection Agency (EPA) registers and regulates disinfectants and chemical sterilants and places them into the categories described in Table 7-2. In dentistry, only those products that are registered with the EPA as hospital disinfectants with tuberculocidal claims (kills the organism Mycobacterium tuberculosis) should be used to disinfect dental treatment areas. M. tuberculosis is highly resistant to disinfectants (Figure 7-5).
TABLE 7-2
| Level of Disinfection | EPA Classification | Use |
| High-level | High-level disinfectant with a relatively short contact time, and a sterilant when used with a prolonged contact time | Semicritical items that cannot tolerate heat sterilization |
| Intermediate-level | Hospital disinfectant with tuberculocidal activity | Noncritical items or surfaces that have been contaminated with blood or saliva |
| Low-level | Nontuberculocidal | Surfaces not contaminated with blood |
Characteristics of Disinfectants
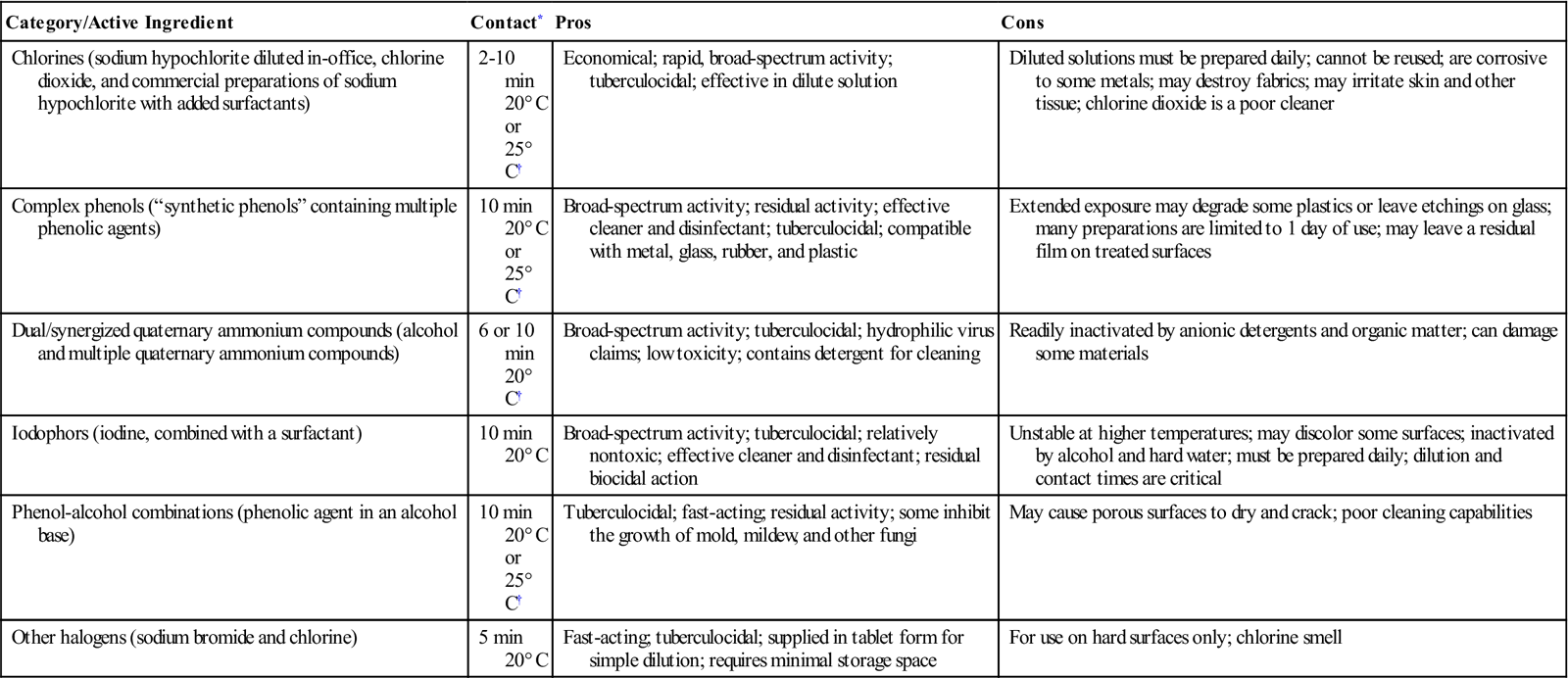
Ideally the perfect disinfectant would be one that would rapidly kill all types of pathogenic organisms, and would be odorless, gentle to dental equipment surfaces, nontoxic, and economical to use. Unfortunately, there is no perfect surface disinfectant; you must make informed choices. Nevertheless, several classes of disinfecting chemicals are available for use in dentistry (Table 7-3).
TABLE 7-3
EPA-Registered Surface Disinfectants for Dentistry
< ?comst?>
| Category/Active Ingredient | Contact* | Pros | Cons |
| Chlorines (sodium hypochlorite diluted in-office, chlorine dioxide, and commercial preparations of sodium hypochlorite with added surfactants) | 2-10 min 20° C or 25° C† |
Economical; rapid, broad-spectrum activity; tuberculocidal; effective in dilute solution | Diluted solutions must be prepared daily; cannot be reused; are corrosive to some metals; may destroy fabrics; may irritate skin and other tissue; chlorine dioxide is a poor cleaner |
| Complex phenols (“synthetic phenols” containing multiple phenolic agents) | 10 min 20° C or 25° C† |
Broad-spectrum activity; residual activity; effective cleaner and disinfectant; tuberculocidal; compatible with metal, glass, rubber, and plastic | Extended exposure may degrade some plastics or leave etchings on glass; many preparations are limited to 1 day of use; may leave a residual film on treated surfaces |
| Dual/synergized quaternary ammonium compounds (alcohol and multiple quaternary ammonium compounds) | 6 or 10 min 20° C† |
Broad-spectrum activity; tuberculocidal; hydrophilic virus claims; low toxicity; contains detergent for cleaning | Readily inactivated by anionic detergents and organic matter; can damage some materials |
| Iodophors (iodine, combined with a surfactant) | 10 min 20° C |
Broad-spectrum activity; tuberculocidal; relatively nontoxic; effective cleaner and disinfectant; residual biocidal action | Unstable at higher temperatures; may discolor some surfaces; inactivated by alcohol and hard water; must be prepared daily; dilution and contact times are critical |
| Phenol-alcohol combinations (phenolic agent in an alcohol base) | 10 min 20° C or 25° C† |
Tuberculocidal; fast-acting; residual activity; some inhibit the growth of mold, mildew, and other fungi | May cause porous surfaces to dry and crack; poor cleaning capabilities |
| Other halogens (sodium bromide and chlorine) | 5 min 20° C |
Fast-acting; tuberculocidal; supplied in tablet form for simple dilution; requires minimal storage space | For use on hard surfaces only; chlorine smell |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
EPA, Environmental Protection Agency.
Note: Glutaraldehydes and simple quaternary ammonium compounds should not be used for surface disinfection in dentistry. High-concentration alcohols (ethyl alcohol or isopropyl alcohol of at least 70%) should be used on precleaned surfaces.
< ?comst1?>< ?comen1?>*< ?comst1?>< ?comen1?>Contact time and temperatures for tuberculocidal activity.
< ?comst1?>< ?comen1?>†< ?comst1?>< ?comen1?>Varies by active ingredient or disinfectant brand.
From Infection control in practice, vol 1, no 3, Annapolis, Md, 2002, OSAP.
Many manufacturers of dental equipment will recommend which surface disinfectants are most appropriate for their dental chairs and unit accessories.
Types of Chemical Disinfectants
Iodophors
Iodophors are EPA-registered, intermediate-level hospital disinfectants with tuberculocidal action. Iodophors are recommended for disinfecting surfaces that have been soiled with potentially infectious patient material. When used according to the manufacturer’s instructions, iodophors are usually effective within 5 to 10 minutes (Figure 7-6). Iodophors are inactivated by hard water, so they must be mixed with soft or distilled water. Because they contain iodine, iodophors may corrode or discolor certain metals or m/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses