Chapter 6
Structure and Physiology of the Periodontium1
Arthur R. Hand
Departments of Craniofacial Sciences and Cell Biology, University of Connecticut, School of Dental Medicine
1 With a contribution by Bradley K. Formaker, Department of Oral Health and Diagnostic Sciences, University of Connecticut, School of Dental Medicine
The periodontium, literally “around the tooth,” comprises the tissues that invest and support the teeth in the maxilla and mandible. The relationship between the teeth and their supporting tissues is complex, involving multiple systems: vascular, neurological, and immunological, to nourish, control, and protect this “joint.” The focus of this chapter is on cementum, the mineralized tissue that covers the root dentin, the periodontal ligament (PDL), the structure that attaches to and holds the tooth in its socket (alveolus), and the alveolar bone, the portion of the jaws that forms the alveolus. The gingivae, the portion of the oral mucosa adjacent to the teeth, also is part of the periodontium; its structure and function are presented in Chapter 9.
Cementum structure, composition and formation
Structure of cementum
Cementum is a collagen-based, mineralized tissue, with similarities to bone. Cementum may be acellular, consisting only of extracellular matrix components and mineral, or it may be cellular, with its forming cells, cementoblasts, trapped within the matrix as cementocytes (Figs. 6.1 and 6.2). Acellular cementum, also called acellular extrinsic fiber cementum, is the first cementum deposited on the dentin of the forming root, and is the predominant form found on the coronal portion of the root. Cellular cementum, also called cellular intrinsic fiber cementum, is most abundant on the apical one-third to one-half of the root and in the furcation areas of multirooted teeth. Cellular cementum may be deposited on top of acellular cementum, especially in response to functional demands and post-eruptive tooth movements. Alternating layers of cellular and acellular cementum may occur (Figs. 6.2 and 6.3); this type of cementum is called cellular mixed stratified cementum. A fourth cementum variety, acellular afibrillar cementum, may be found near the cervical margin, covering small areas of enamel and dentin. Acellular afibrillar cementum has a mineralized matrix that lacks collagen fibrils.
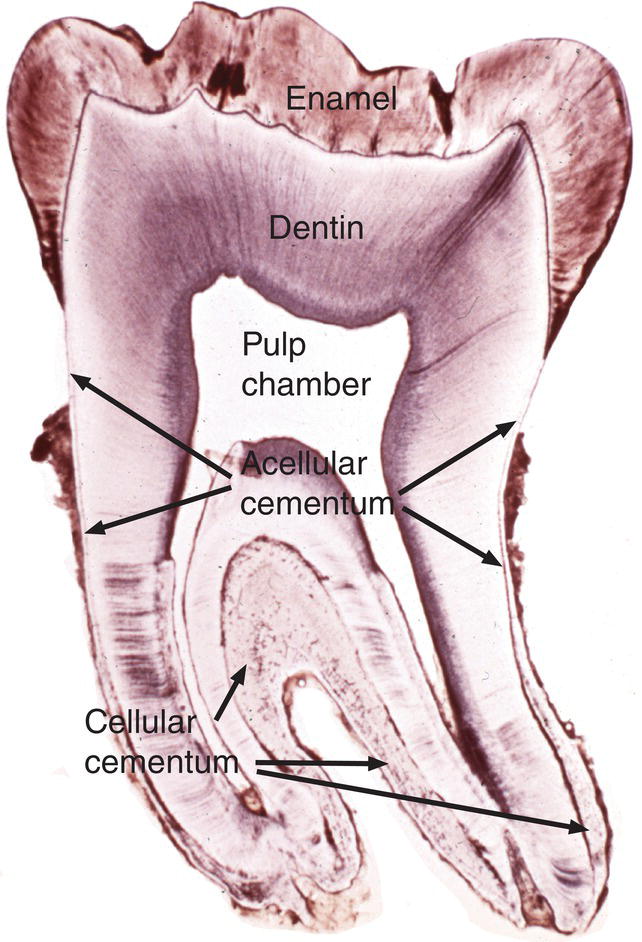
Figure 6.1 Cementum location. Low magnification image of a ground section of a mandibular molar tooth. Cementum covers the surface of the root dentin. Acellular cementum is located on the coronal one-half to two-thirds of the root; cellular cementum is located on the apical one-third to one-half of the root and in the furcation area between the two roots. Note that cellular cementum is considerably thicker than acellular cementum.
(From Moss-Salentijn, L. (1972) Orofacial Histology and Embryology: A Visual Integration. Reproduced with permission from F.A. Davis Company, Philadelphia, PA.)
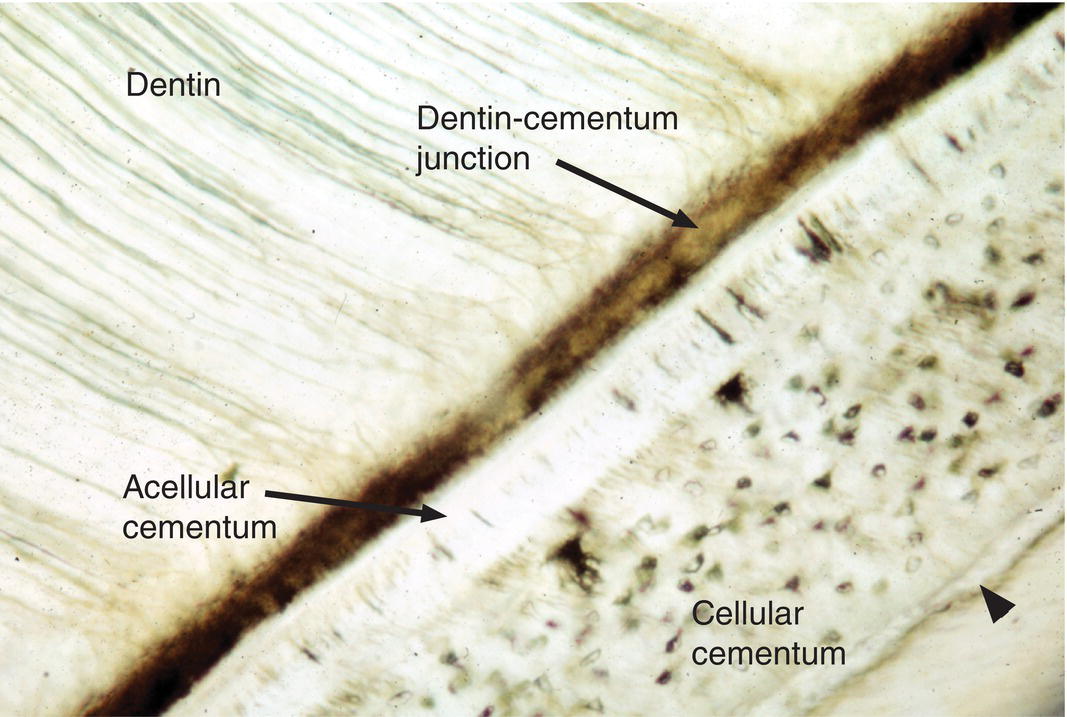
Figure 6.2 Cellular and acellular cementum. Ground section from the apical portion of a root. A layer of acellular cementum adjacent to the dentin is covered by a thicker layer of cellular cementum containing cementocyte lacunae. A thin layer of acellular cementum (arrowhead) is present on the surface of the cellular cementum.
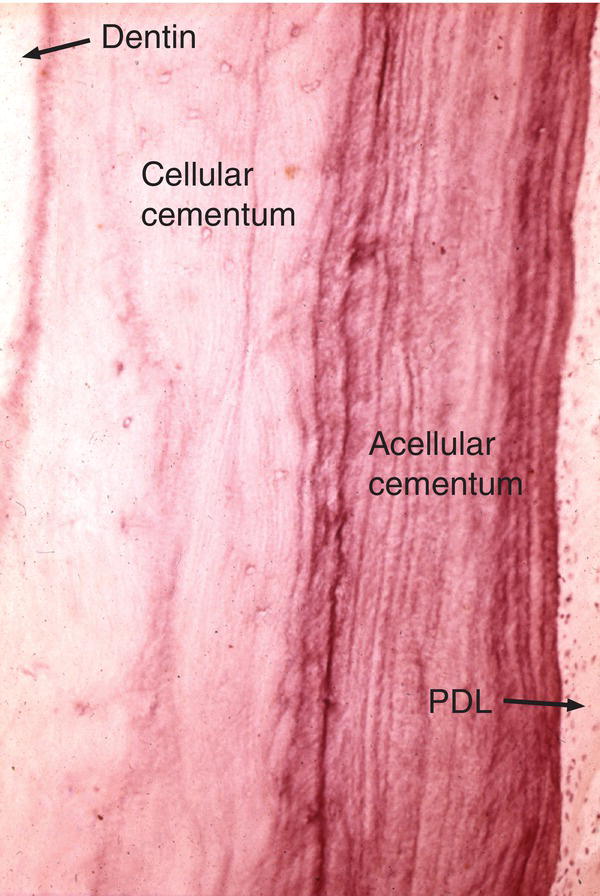
Figure 6.3 Cellular and acellular cementum. A thick layer of cellular cementum is covered by acellular cementum with many incremental lines. Periodontal ligament (PDL).
Cementum, unlike bone, is avascular, lacks innervation and undergoes little remodeling. Its nutrition comes by diffusion from nearby blood vessels in the periodontal ligament. In regions where the cementum is very thick, such as the root apices or furcation areas, cementocytes in the deeper layers may not receive sufficient nutrition to survive. The cementocytes, like osteocytes of bone, are located within lacunae and have long cellular processes present in canaliculi that extend toward the periodontal ligament (Fig. 6.4).
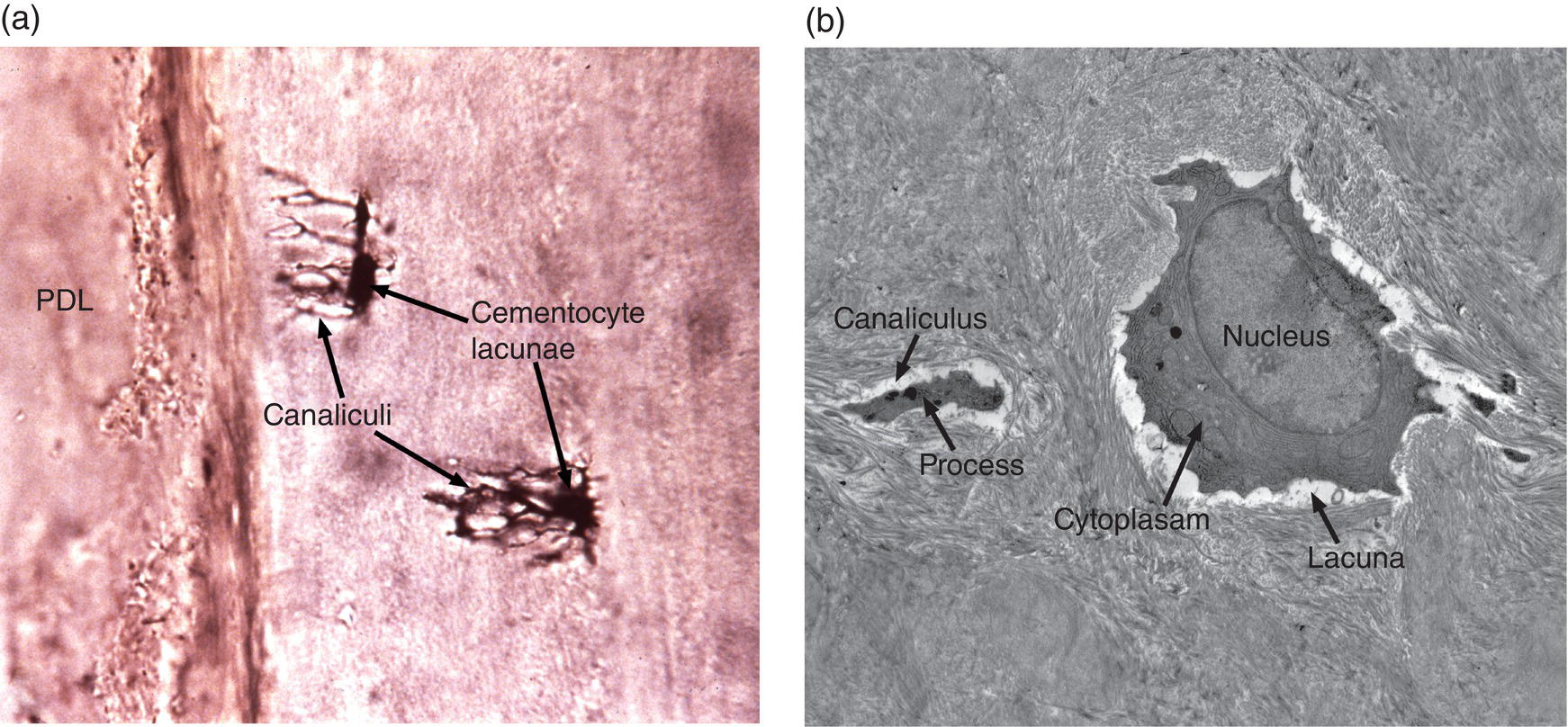
Figure 6.4 Cementocytes. (a) Ground section of cellular cementum showing cementocyte lacunae with canaliculi extending toward the cementum surface and the site of the periodontal ligament. (b) Electron micrograph of demineralized cellular cementum of a mouse molar tooth showing a cementocyte in a lacuna surrounded by the collagenous cementum matrix. One of the cementocyte processes within a canaliculus is indicated.
(a) From Moss-Salentijn, L. 1972. Orofacial Histology and Embryology: A Visual Integration. Reproduced with permission from F.A. Davis Company, Philadelphia, PA.)
Composition of cementum
The composition of cementum is shown in Table 6.1. Cementum consists of about 45 to 50% mineral (hydroxyapatite) and about 45 to 50% organic material and water. Type I collagen accounts for about 90% of the organic matrix of cementum. Other constituents include several proteins also found in bone and/or dentin, growth factors, and cementum-specific proteins.
Table 6.1 Composition of cementum
| Constituent | Properties and function |
| Hydroxyapatite | Hardness |
| Collagen I | Tensile strength; substrate for mineralization |
| Other collagens (III, V, VI, XII, XIV) | Associated with collagen I |
| Non-collagenous proteins | |
| Tissue nonspecific alkaline phosphatase | Hydrolysis of pyrophosphate and dephosphorylation of osteopontin, both inhibitors of mineralization |
| Bone sialoprotein | Recruitment and adhesion of cells to root surface; mineralization initiator |
| Dentin matrix protein 1 (DMP-1) | Regulation of mineralization |
| Dentin sialoprotein | Regulation (inhibition?) of mineralization |
| Fibronectin | Cell adhesion |
| Osteocalcin | Regulation of mineralization |
| SPARC/Osteonectin | Collagen binding; regulation of collagen content and fibril size; modulation of growth factor signaling |
| Osteopontin | Recruitment and adhesion of cells to root surface; mineralization inhibitor |
| Proteoglycans | Regulation of cell-cell and cell-matrix interactions; regulation of mineralization; binding of growth factors |
| Proteolipids | Cell membrane constituents |
| Tenascin | Cell adhesion |
| Cementum attachment protein | Cementoblast proliferation and differentiation |
| Cementum protein 1 | Proliferation and differentiation of PDL cells toward cementoblast, osteoblast, and chondroblast lineages |
| Growth factors (insulin-like growth factor-1, fibroblast growth factors -1, -2, platelet-derived growth factor, transforming growth factor-ß, bone morphogenetic proteins -2, -3, -4, epidermal growth factor) | Promotion of cell proliferation; growth and differentiation; matrix synthesis |
Formation of the tooth root
Initial cementum formation occurs concomitantly with root formation. Upon completion of the crown, the cervical loop of the enamel organ, consisting of the inner and outer enamel epithelia, exhibits significant cell proliferation and grows in an apical direction, forming Hertwig’s epithelial root sheath and the epithelial diaphragm (Figs. 6.5a and 6.5b). At the advancing apical extent of the developing root, the cells of the epithelial root sheath lie adjacent to the ectomesenchymal cells of the dental papilla, separated only by a basal lamina. The root sheath cells induce the adjacent dental papilla cells to differentiate into odontoblasts. Following secretion of the initial root predentin, the epithelial root sheath becomes discontinuous and the epithelial cells migrate away from the root surface (Fig. 6.6a). Components of their basal lamina, such as the glycoprotein laminin and possibly small amounts of enamel matrix proteins, are thought to serve as chemoattractants for dental follicle cells, which migrate in to contact the surface of the root predentin. As described below, these cells from the dental follicle initiate the formation of acellular cementum.
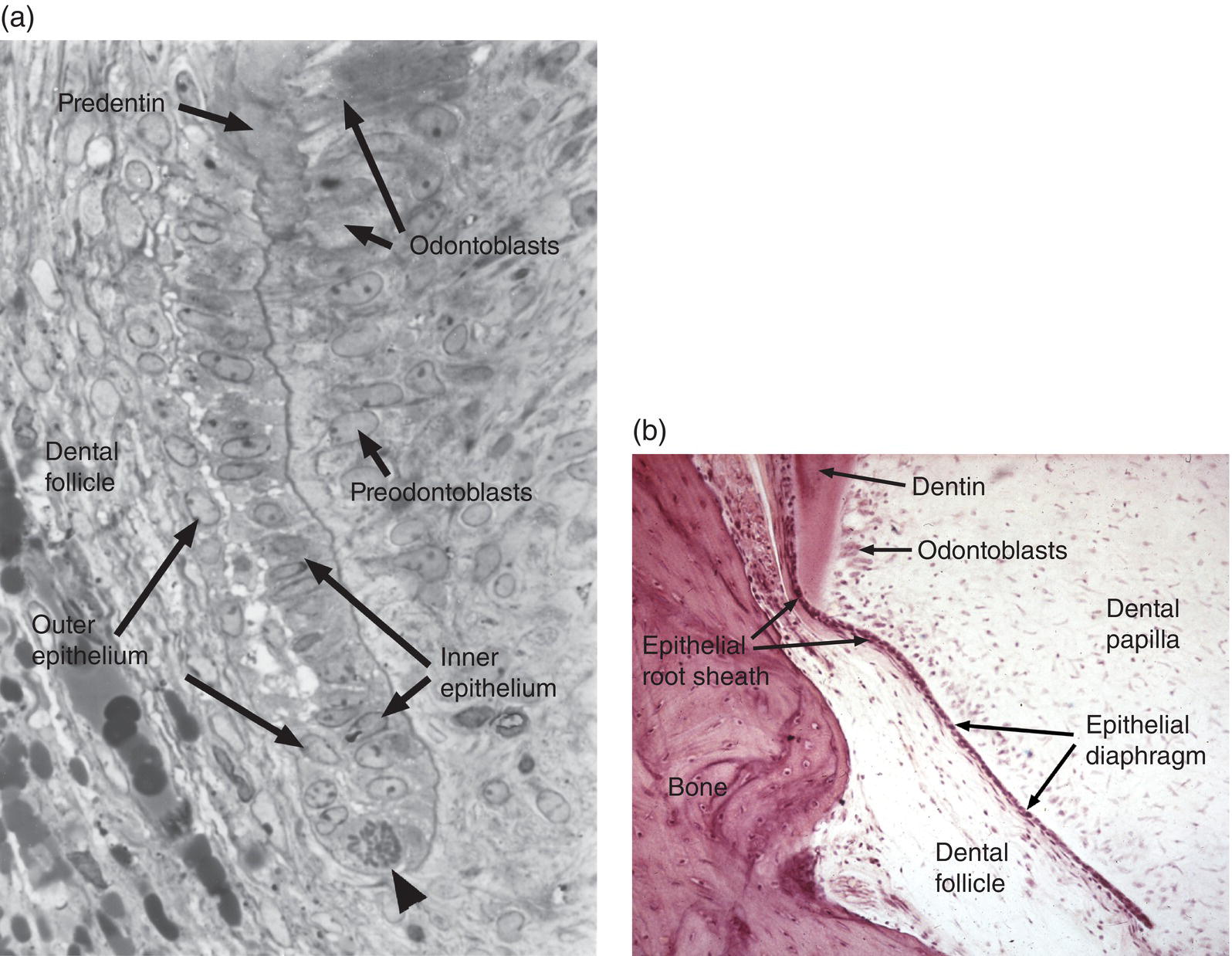
Figure 6.5 Epithelial root sheath and epithelial diaphragm. (a) The epithelial root sheath consists of two cell layers derived from fusion of the inner and outer enamel epithelia. The root sheath induces differentiation of root odontoblasts from the dental papilla cells. A mitotic cell (arrowhead) indicates the growth of the root sheath at its apical tip.
(From Bosshardt, D.D. & Selvig, K.A. (1997) Dental cementum: the dynamic tissue covering of the root. Periodontology 2000, 13, 41–75. Reproduced by permission of John Wiley and Sons.) (b) The epithelial diaphragm is a continuation of the root sheath beneath the dental papilla. The diaphragm determines the formation of single versus multiple roots.
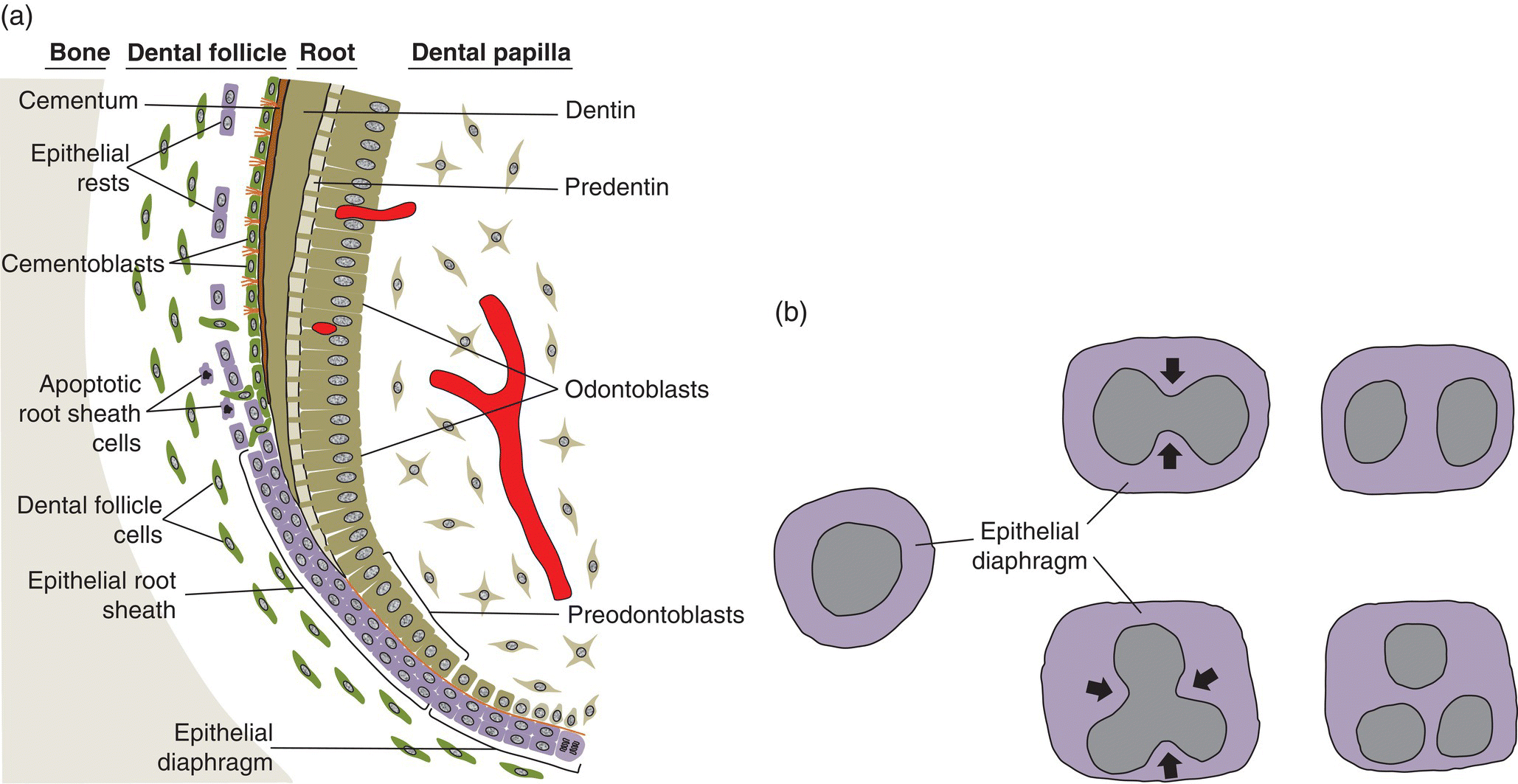
Figure 6.6 Epithelial root sheath and diaphragm. (a) The two-cell layer thick epithelial root sheath grows apically from the cervical loop of the former enamel organ and induces mesenchymal cells of the dental papilla to differentiate into pre-odontoblasts and odontoblasts. After odontoblasts secrete the predentin matrix, the root sheath fragments and the cells migrate away from the root surface. Some root sheath cells undergo apoptosis, whereas others remain in the dental follicle (and subsequently the periodontal ligament) as a network of epithelial cell rests. As the root sheath fragments, dental follicle cells migrate to the root surface, differentiate into cementoblasts, and begin deposition of cementum and formation of a fringe of collagen fibrils (orange fibers in diagram). Some root sheath cells may transform into mesenchymal cells, which differentiate to cementoblasts (not shown). (b) The epithelial diaphragm seen in an apical view of developing teeth with a single root (left) and two or three roots (center and right). In multirooted teeth, the epithelial diaphragm grows inward (arrows) at two or three sites and fuses to delineate the roots.
The two-cell-layer thick epithelial diaphragm extends inward beneath the dental papilla (Fig. 6.5b), separating the dental papilla from the dental follicle. The epithelial diaphragm serves to delineate the morphology of the developing root. In multirooted teeth, the diaphragm grows together at specific points to create the furcation, or branch point, between the roots (Fig. 6.6b).
After the initiation of root dentin formation and migration away from the root surface, the epithelial root sheath cells have three possible fates.
- Some cells undergo apoptosis.
- Some cells remain viable and may exist within the periodontal ligament for as long as the tooth remains in the alveolus. These cells, called the epithelial rests of Malassez, typically are seen in small clusters or short strands extending along and close to the root surface (Fig. 6.7a). In sections parallel to the root surface, the epithelial rest cells are seen to form a network of branching and anastomosing strands (Fig. 6.7b), probably a result of the apoptotic death of some cells. Although the number of epithelial rest cells is reduced with age, mitotic activity has been observed in these cells. It is not known if the epithelial rest cells have a specific function in the periodontal ligament.
- Some root sheath cells may undergo transformation to mesenchymal cells, which then differentiate to cementoblasts. Such epithelial-mesenchymal transformations occur during development of other craniofacial structures, such as the transformation of ectodermal neural crest cells to craniofacial mesenchymal cells. These transformed epithelial root sheath cells may deposit acellular or cellular cementum, and may be incorporated as cementocytes in cellular cementum.
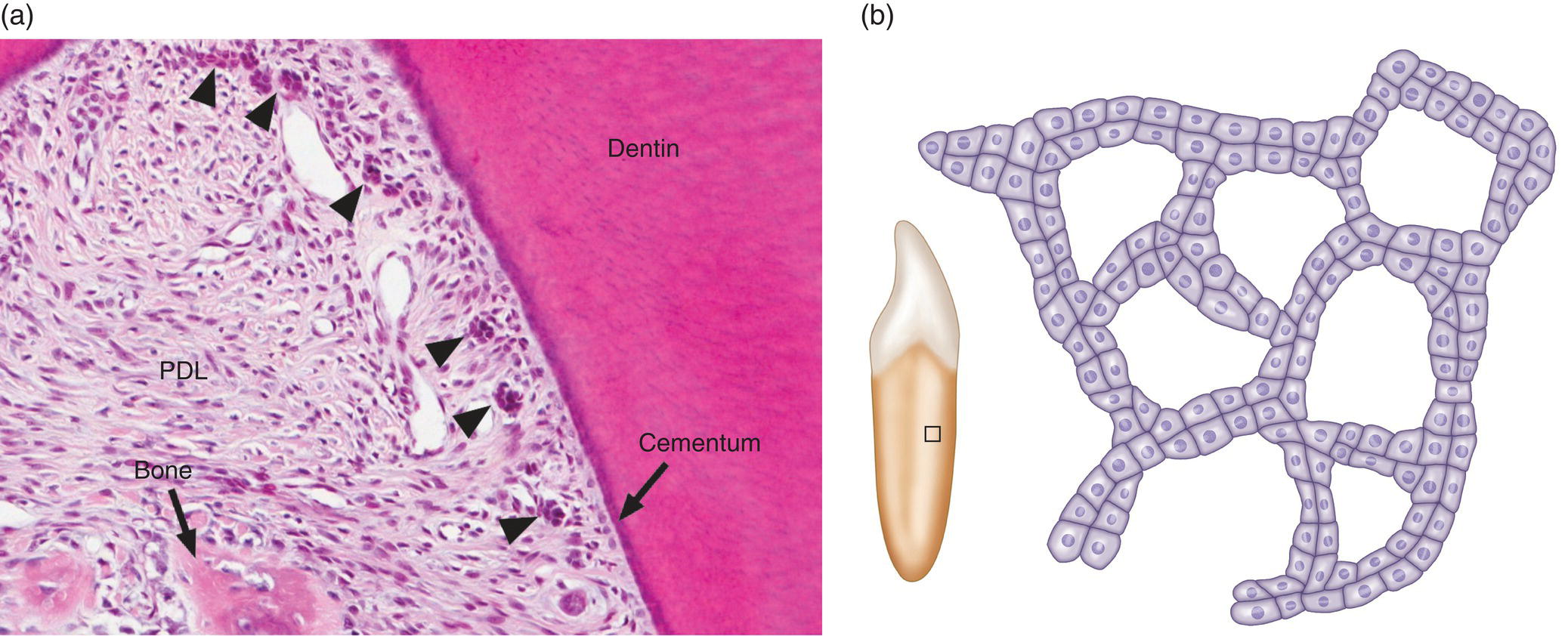
Figure 6.7 Epithelial rests of Malassez. (a) Light micrograph of the furcation region of the first permanent molar of a young child. Several clusters of epithelial rest cells (arrowheads), remnants of the epithelial root sheath, are present along the root surface. The crest of the interradicular bony septum is indicated. Periodontal ligament (PDL). (b) Drawing of the distribution of epithelial rest cells on the root surface. The rests form a network in the periodontal ligament that parallels the root surface. The box on the root of the tooth shows the approximate location of the main drawing.
Cementum formation
The formation of cementum occurs over an extended period of time. Depending upon the tooth, the prefunctional phase, before the tooth reaches the occlusal level, occurs over a period of about 3-1/2 years to almost 8 years. The deposition of acellular cementum occurs at a slow but constant rate of about 3 μm per year on single-rooted teeth; the rate varies, however, with tooth type and root surface area. The postfunctional phase, when the tooth becomes attached to bone via the periodontal ligament, lasts for the life of the tooth.
As the epithelial root sheath begins to break up and move away from the predentin surface, cells with the appearance of fibroblasts, originating from the dental follicle, migrate in between the epithelial cells and differentiate to cementoblasts. The expression of bone morphogenetic protein 3 (BMP-3) by dental follicle cells, and later by cells lining the root surface, has been linked to the differentiation of cementoblasts. These newly differentiated cementoblasts begin to deposit collagen fibrils oriented perpendicular to the root surface (Figs. 6.8 and 6.9). This fringe of collagen fibers intermingles with the collagen fibrils of the predentin, forming a firm attachment at the dentin-cementum junction. Mineralization of the mantle predentin begins internally and proceeds in a peripheral direction, encompassing the dentin-cementum junction and spreading into the cementum. The cementoblasts on the root surface continue to deposit collagen, lengthening the fiber fringe, and also secrete non-collagenous proteins around the fibrils. When this initial layer of cementum achieves a thickness of 15 to 20 μm, at about the time that the cusp tip penetrates the oral mucosa, the fiber fringe connects with the principal fiber bundles of the developing periodontal ligament (Fig. 6.9). Acellular cementum thus serves as the main site of attachment of periodontal ligament collagen fibers. These fibers, inserting roughly perpendicularly into the cementum, are visible in histologic sections and are called Sharpey’s fibers (Fig. 6.10).
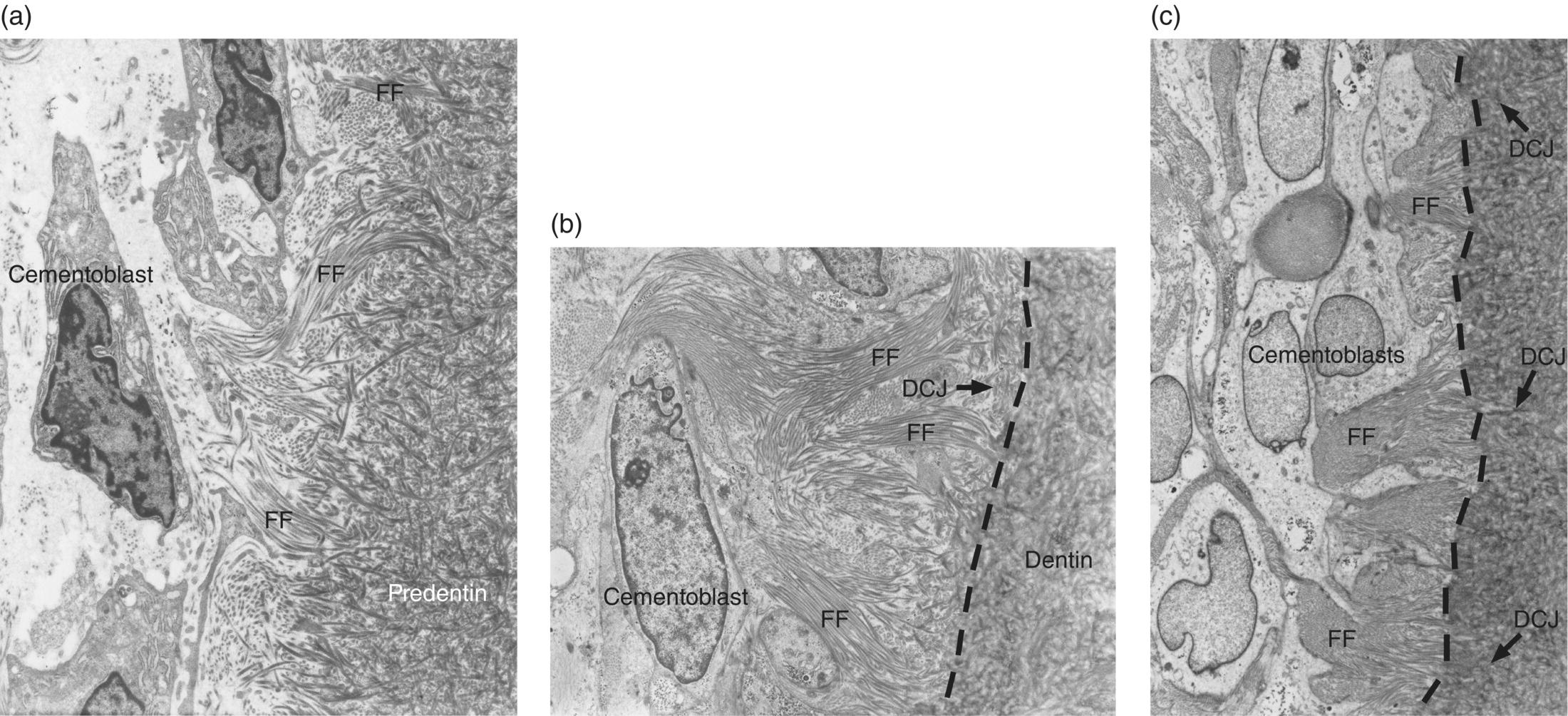
Figure 6.8 Electron micrographs showing development of the initial acellular cementum matrix and the dentin-cementum junction. (a) Cementoblasts on the root surface produce and implant collagen fibrils into the predentin, forming a fiber fringe (FF) oriented roughly perpendicular to the developing root. (b) Cementum matrix almost completely covers the predentin matrix, and the external mineralizing front (dashed line) of the dentin has almost reached the dentin-cementum junction (DCJ). (c) The fiber fringe extends into the space of the immature periodontal ligament. The mineralization front (dashed line) of the dentin has passed the dentin-cementum junction.
((a), (b), and (c) From Bosshardt, D.D. & Selvig, K.A. (1997) Dental cementum: the dynamic tissue covering of the root. Periodontology 2000, 13, 41–75. Reproduced by permission of John Wiley and Sons.)
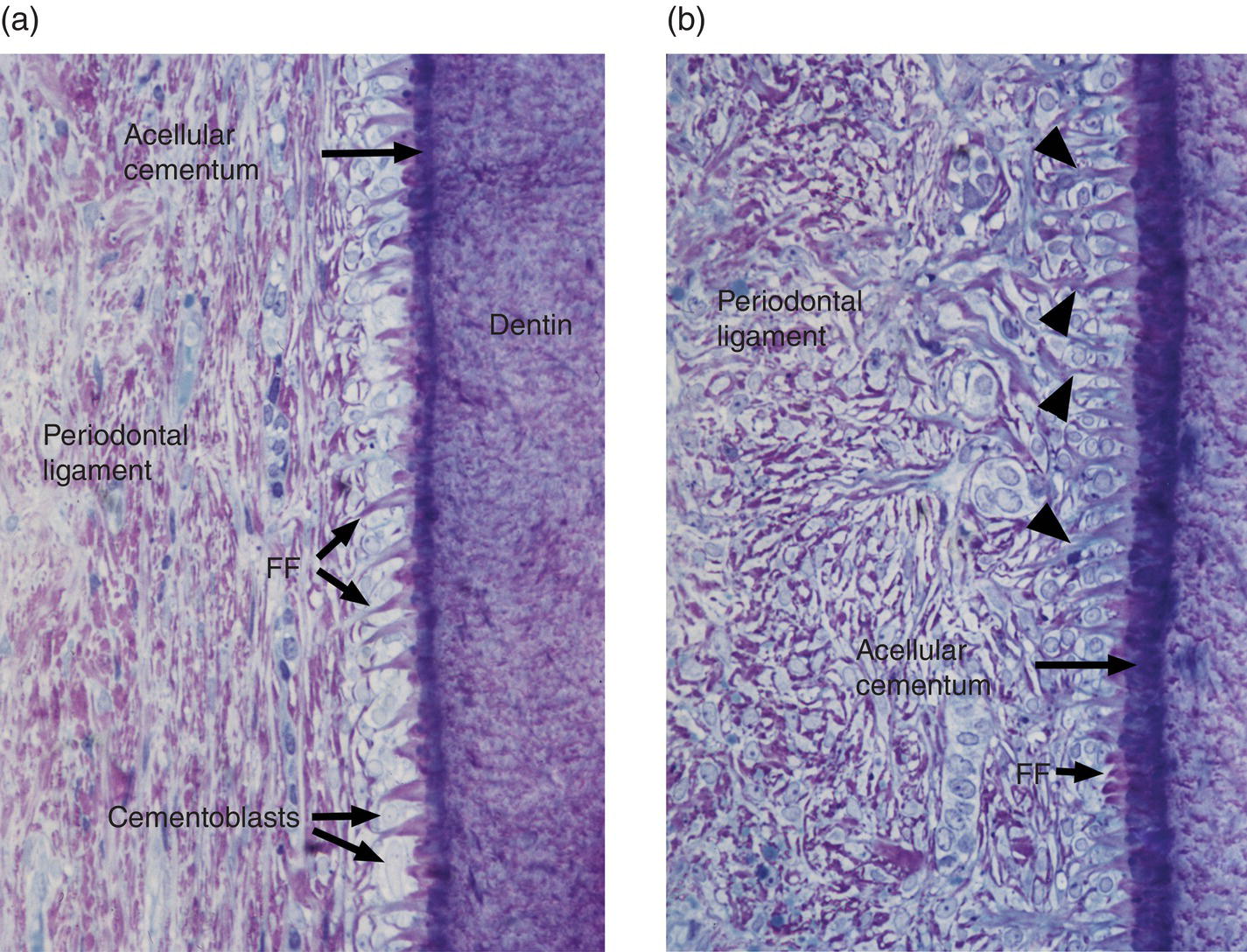
Figure 6.9 Light micrographs of developing cementum and periodontal ligament of unerupted human premolar teeth. (a) A fiber fringe (FF) protrudes from a thin layer of mineralized acellular cementum. At this stage the periodontal ligament consists of a fibrocellular meshwork oriented parallel to the root surface. (b) A 15-μm-thick layer of mineralized cementum covers the root surface. The orientation of the periodontal ligament fibers has changed; many are now roughly perpendicular to the root. Some of the periodontal ligament fibers are continuous with the fiber fringe (arrowheads).
((a) and (b) From Bosshardt, D.D. & Selvig, K.A. (1997) Dental cementum: the dynamic tissue covering of the root. Periodontology 2000, 13, 41–75. Reproduced by permission of John Wiley and Sons.)
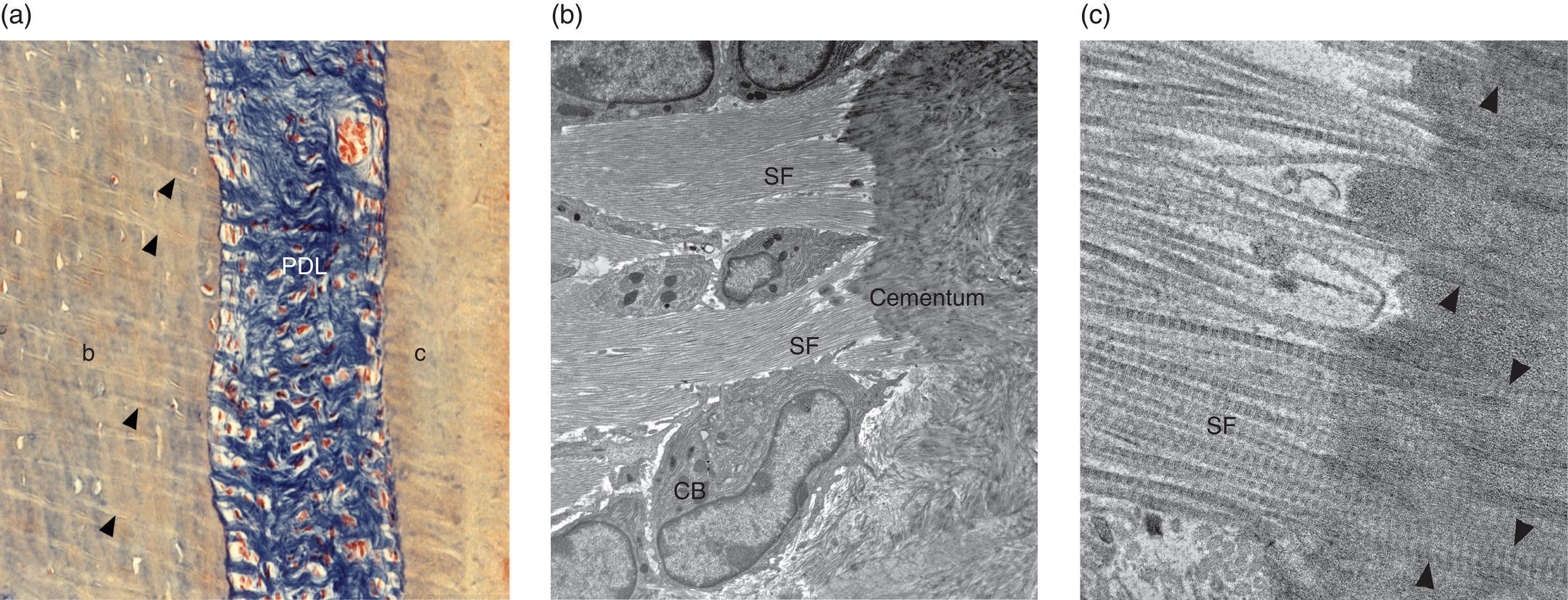
Figure 6.10 Sharpey’s fibers. (a) Light micrograph of human periodontal ligament Sharpey’s fibers. The collagen fibers of the periodontal ligament (PDL) are stained blue. Sharpey’s fibers (arrowheads) are embedded in the alveolar bone (b). Sharpey’s fibers also are present in cementum (c) but are less distinct. Azan stain. (b) and (c) Electron micrographs of periodontal ligament fibers inserting into cementum (Sharpey’s fibers, SF) of a mouse molar tooth. (a) The Sharpey’s fibers pass between cementoblasts (CB) to enter the cementum. (b) At high magnification the individual banded collagen fibrils of the Sharpey’s fibers can be seen in the cementum (arrowheads).
(From: Beertsen, W., McCulloch, C.A.G., & Sodek, J. (1997) The periodontal ligament: a unique, multifunctional connective tissue. Periodontology, 2000, 13, 20-40. Reprinted with permission from John Wiley and Sons.)
Cellular cementum forms rapidly, up to 30 times faster than acellular cementum. Newly differentiated cementoblasts cover the root predentin in the apical and furcation regions and begin to deposit the initial matrix of cementum, with collagen fibrils intermingling with those of the predentin. Mineralization of the predentin extends across the dentin-cementum junction into the newly formed cementum. An irregular layer of unmineralized cementum, precementum or cementoid, is present between the cementoblasts and the mineralization front. Cementoblasts secrete matrix components completely around themselves, resulting in their entrapment in lacunae. The collagen fibrils of cellular cementum are mainly oriented parallel to the root surface, running in a circular direction around the tooth. Cellular cementum is deposited mainly as an adaptive tissue, in response to functional demands and to repair resorptive defects. Sharpey’s fibers are fewer in number and more irregularly spaced in cellular cementum than in acellular cementum.
Cementum mineralization shares similarities with, but also differs from, the mineralization process in bone. For example, there is no evidence for a role of matrix vesicles in acellular cementum mineralization; mineral crystals are first deposited within the collagen fibrils, then between the fibrils (Fig. 6.11). Cementoblasts regulate the extracellular levels of the mineralization inhibitor inorganic pyrophosphate (PPi) in order to control the amount of cementum deposition. Regulation of inorganic pyrophosphate levels is critical for proper acellular cementum formation, but less so for cellular cementum. Cementum mineralization is a very slow process; the width of the unmineralized precementum layer is 3 to 5 μm, roughly equivalent to the yearly deposition of cementum, and mineral crystals achieve their mature size between 1 and 4 μm deep to the mineralization front.
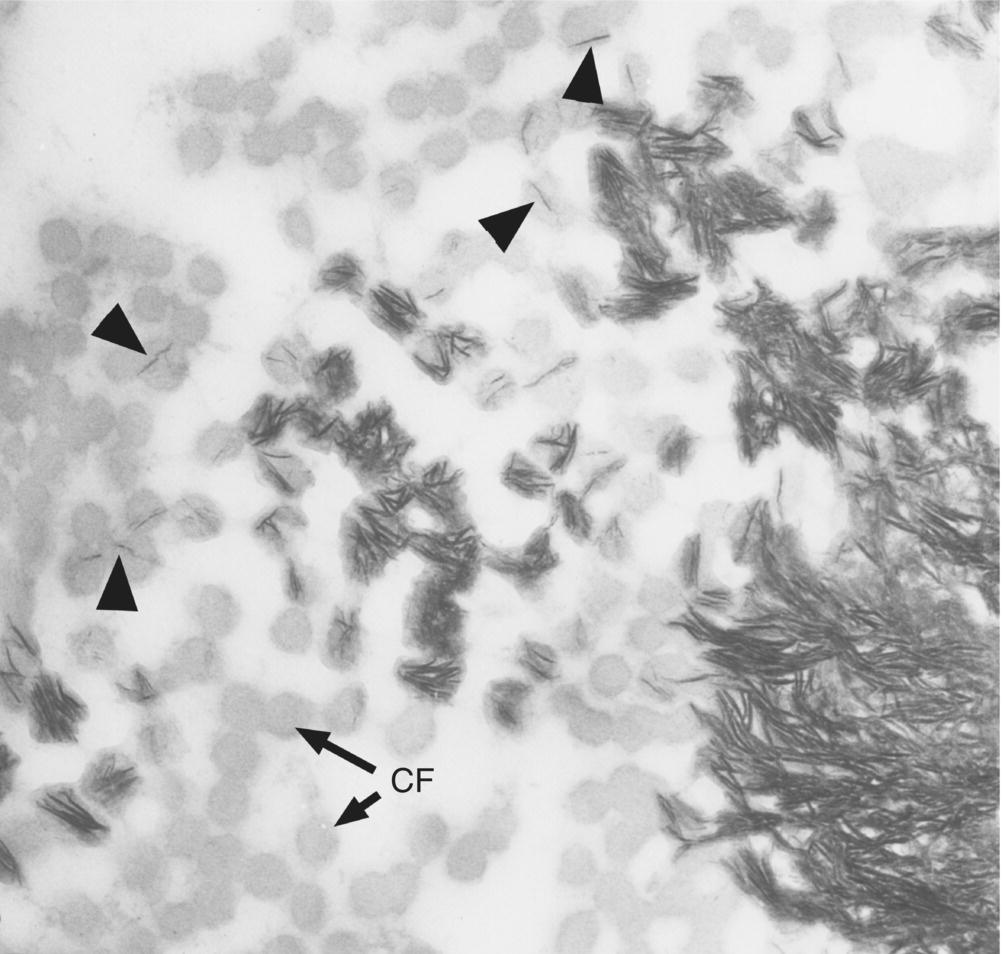
Figure 6.11 Electron micrograph of a section parallel to the root surface at the mineralization front of cementum. Cross-sectioned collagen fibrils (CF) appear as gray circles. Individual mineral crystals (fine dark lines) form within the collagen fibrils (arrowheads); additional mineral crystals are deposited within the fibrils (center), and finally between the fibrils to form the completely mineralized cementum (lower right).
(From Bosshardt, D.D. & Selvig, K.A. (1997) Dental cementum: the dynamic tissue covering of the root. Periodontology 2000, 13, 41–75. Reproduced by permission of John Wiley and Sons.)
Tension transmitted through the periodontal ligament appears to stimulate cementum deposition. During orthodontic treatment, new cementum formation is greater on the tension side of the root than on the pressure side (the side toward which the tooth is being moved). Also, as a result of the normal mesial drift of teeth in the arch, cementum is thicker on the distal surface of the root than on the mesial surface. Cementum deposition, primarily cellular cementum, also occurs during repair of resorption defects (Fig. 6.12).
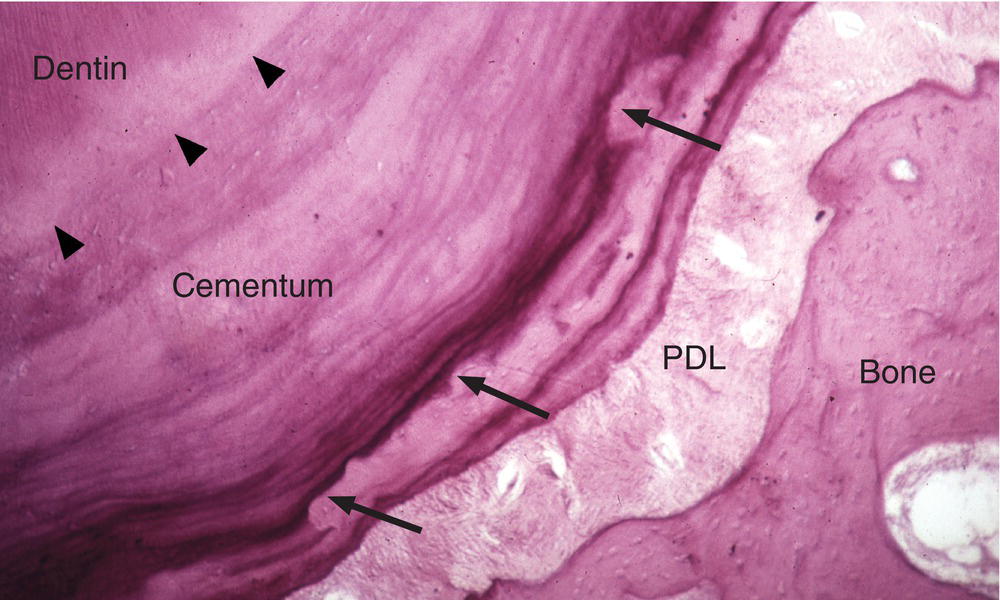
Figure 6.12 Cementum resorption and repair. Resorption of cementum by odontoclasts (similar to osteoclasts) may occur in a variety of conditions, including during orthodontic tooth movement. Resorbed areas (arrows) may be filled in later with newly deposited cementum. Arrowheads indicate the dentin-cementum junction.
Periodontal ligament structure and function
The periodontal ligament attaches the tooth root to alveolar bone (Fig. 6.13), and it serves to absorb and resist the forces of occlusion on the tooth. It consists of collagenous fiber bundles containing hundreds or thousands of individual collagen fibrils (Fig. 6.14). The collagen fibrils and other extracellular matrix components are synthesized and maintained by periodontal ligament fibroblasts. Type I collagen is the major constituent of the fibers, but types III and XII collagen also are present. Type III collagen synthesis increases during periodontal ligament remodeling, whereas type XII collagen is thought to be important in maintaining the organization of the collagen fiber bundles. The fibroblasts are aligned along and between the fiber bundles and extend cytoplasmic processes that surround and organize the bundles. The periodontal ligament fiber bundles are embedded, as Sharpey’s fibers, in cementum on the root and in the alveolar bone facing the tooth (Fig. 6.15). The embedded portions of Sharpey’s fibers are fully mineralized in acellular cementum and partially mineralized in cellular cementum and bone. Interstitial areas containing loose connective tissue, blood vessels, and nerves are present between the fiber bundles in the periodontal ligament. These interstitial areas are continuous with openings through the alveolar bone (Volkmann’s canals) to the marrow spaces of the alveolar process (Fig. 6.13).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


