6 Oral Surgery for the General Practitioner
The specialty of oral and maxillofacial surgery (OMS) has benefited from the use of lasers since the mid-1960s,1 with the first documented use of a laser in OMS in 1977.2 Lasers are quickly becoming the standard of care for many surgical procedures, given the advantages of improved visualization, hemostasis, and reduced discomfort. The introduction of lasers that incorporate computer interface technology has made lasers much more “user friendly,” contributing to their popularity in the dental profession. Manufacturers are also addressing the need for mobility and designing lighter, more portable equipment, making lasers easier to maneuver from room to room. Laser handpieces have interchangeable components that are more ergonomic and versatile, allowing better control when performing exacting procedures in the confines of the oral cavity.
Intraoral Lasers
Understanding laser physics and laser light’s biological interaction with tissues is essential when determining the appropriate laser for any given procedure. A wide array of lasing media with unique radiant-energy wavelengths has been employed successfully for varying indications and types of tissue, including the argon (Ar), carbon dioxide (CO2), erbium:yttrium-aluminum-garnet (Er:YAG) and erbium, chromium:yttrium-scandium-gallium-garnet (Er,Cr:YSGG), holmium:YAG (Ho:YAG), neodymium:YAG (Nd:YAG), potassium titanyl phosphate (KTP), pulsed dye, and diode lasers.3 The diode, Nd:YAG, erbium, and CO2 lasers are the most common intraoral lasers because of their wavelength-dependent properties (see Chapter 2).
Diode Laser (805-1064 nm)
Many manufacturers produce diode lasers, delivering wavelengths in the range of 805 to 1064 nm. They are compact and portable in design and are relatively inexpensive surgical units, with efficient and reliable benefits for use in soft tissue oral surgical procedures. Diode lasers can be used in continuous-wave or gated-pulse modes, in contact or out of contact with tissue. The 980-nm diode laser has significantly higher absorption in water, which makes it cut more optically than thermally, with an optical penetration of less than 300 microns (µ, µm). Romanos and Nentwig4 found that the 980 nm diode laser produced a more precise incision margin compared with other laser wavelengths. In addition to various soft tissue oral surgical procedures, 980-nm diode lasers have become as popular as CO2 lasers in the treatment of periimplantitis because they offer a bactericidal effect without causing implant surface alterations5 (see Chapter 7).
Neodymium:YAG Laser (1064 nm)
The Nd:YAG laser’s active medium is a crystal of yttrium, aluminum, and garnet doped with neodymium ions.6 By functioning in the near-infrared part of the spectrum at 1064 nm, the Nd:YAG laser exhibits minimal surface tissue absorption and maximal penetration; this allows for coagulation of tissue in depth.7 The optical delivery is free-running but must be in the “pulsed” mode because of Nd:YAG’s ability to penetrate deeply into soft tissues. Romanos8 believed that most procedures could be performed without local anesthesia because the pulse duration is shorter than the time required to initiate a nerve action potential.
Comparing Nd:YAG laser surgery to conventional scalpel surgery, White et al.9 concluded that the laser could be used successfully for intraoral soft tissue applications without anesthesia and with minimal bleeding. When the procedure involves significant ablation or resection of tissue, local anesthesia is necessary for patient comfort.7
As with the diode lasers, the Nd:YAG laser can be used in a contact (excision) and a noncontact (coagulation) mode. These properties have led to its use in a variety of maxillofacial procedures, including coagulation of angiomatous lesions, hemostasis in bleeding disorders, arthroscopic surgery of the temporomandibular joint (TMJ), resections in vascular tissues (in combination with CO2 wavelength), and palliation of advanced neoplasms.10 The Nd:YAG laser has demonstrated some benefit with minimally invasive periodontal therapies, including sulcular debridement and bacterial decontamination, resulting in potential new attachment of gingival tissues, regeneration of supporting bone, and regrowth of periodontal ligament11 (see Chapters 3 to 5).
Erbium Lasers (2780-2940 nm)
The erbium family of lasers, including two similar wavelengths, has gained popularity in dental implant surgery because of several properties. The erbium lasers are free-running pulsed lasers with thermal effects that interact solely with the surface layers of soft and hard tissue.12 The beams are reflected by polished metal surfaces such as titanium, so they have no adverse effects on dental implants.13 Application of the erbium lasers in dental implant surgery has been advocated for the preparation of hard tissue, second-stage surgery, revision of soft tissue, and treatment of periimplantitis.14–16
Carbon Dioxide Laser (10,600 NM)
The CO2 laser has become the workhorse for intraoral soft tissue surgery. The emitted wavelength of 10,600 nm has ideal absorption by soft tissue because soft tissue is composed of 90% water, and CO2 has excellent absorption in water. Cellular rupture occurs from the photothermal effect when intracellular water absorbs the energy from the CO2 laser. The cellular vaporization is the basis for the CO2 laser to function as a surgical tool.3 The wavelength allows for absorption into soft tissue, very quickly generating heat conducted into the surrounding tissue, and creates a very narrow zone of thermal necrosis approximately 500 µ or less.17 Lateral thermal damage of such a small area is an excellent advantage of CO2 laser use, because it results in coagulation of vessels up to 500 µ in diameter and is clinically manifested by hemostasis and sealing of the lymphatics, which has been found to reduce postsurgical bacteremia compared with other methods of incision.18
The learning curve tends to be slightly higher with the CO2 laser because it is the only soft tissue laser used without direct tissue contact. The delivery system is either an articulated arm or a hollow waveguide. The hollow waveguide requires the use of higher power because significant laser energy is absorbed internally by the laser delivery system. These delivery systems are acceptable when one has direct vision in the confines of the oral cavity. However, when performing endoscopic and microscopic surgical procedures in which clinicians are challenged by limited visibility of an area, a flexible core system can provide a needed advantage. Development of the BeamPath fiber allows laser energy to be transmitted by an innovative photonic band-gap omnidirectional dielectric mirror lining, which guides the light through a flexible hollow core.19
Advantages and Disadvantages of Laser Surgery
Benefits
Examined histologically, laser wounds have been found to contain a significantly lower number of myofibroblasts.20 This results in less wound contracture or scarring and, ultimately, improved healing.21,22 Mobility to the dynamic tissues (lips, tongue, floor of mouth, soft palate) is attained more readily postsurgically. As a result of improved healing and hemostasis, intraoral laser wounds can often be left without sutures, healing by secondary intention, except when cosmesis is a concern.
With laser technology, the patient typically experiences less postoperative swelling and pain.23,24 A compromised airway during oral surgery is less of a concern, partly because of the decreased swelling. Although not always predictable, the decreased postoperative pain can be managed with over-the-counter (OTC) nonnarcotic oral analgesics (e.g., ibuprofen) for most laser procedures. The physiology of this effect is still unknown but most likely correlates with decreased tissue trauma and an alteration of neural transmission.7
Drawbacks
Despite the laser’s many advantages, when determining the appropriate treatment for a patient, the clinician also must consider its drawbacks. Although the healing process following laser surgery is generally marked by decreased scarring and increased function, some have found that the speed of healing is slightly prolonged compared with other types of wounds.25 This delay in healing is undoubtedly caused by the sealing of blood vessels and lymphatics and subsequent need for neovascularization in healing. Typical intraoral healing after laser surgery may take as long as 2 weeks for wounds that would otherwise take 7 to 10 days. If sutures are indicated, delayed healing times must be taken into account when considering suture removal, to prevent premature dehiscence of the wound.24
Nonsutured CO2 laser wounds heal forming a fibrinous coagulum that functions as a biological dressing. Because of the slow epithelialization of CO2 laser wounds, the fibrinous coagulum may be present beyond 2 weeks.23 A clinician new to lasers should not confuse this healing process for infection and unnecessarily perform a wound debridement or prescribe antibiotics when there is no indication. Unlike postsurgical healing after conventional techniques, an increase in pain 4 to 7 days postoperatively may occur, which can normally be controlled with minimal oral analgesics (e.g., ibuprofen). The clinical appearance and anticipated discomfort after laser surgery should be discussed with patients to avoid confusion or perceived complications.24
Virtually all laser wavelengths used for surgery to vaporize, coagulate, or cut tissue may produce particulate debris called laser plume. Clinicians, assistants, and patients may be at risk from exposure to laser plume. Laser plume may contain carcinogens, irritants, dusts, viruses, and bacterial spores, depending on the procedure. It may also contain carbon monoxide, polyaromatic hydrocarbons, various toxic gases, and chemicals such as formaldehyde, hydrogen cyanide, and benzene. There are currently no known potential chronic health effects from long-term exposure to laser plume. The literature on infectious transmission of laser plumes in medicine is equivocal. Several studies of patients positive for human papillomavirus type 2 (HPV-2) deoxyribonucleic acid (DNA), revealed no viable viral particles in the laser plume.26–31 Other studies have shown HPV DNA in the plume of laser-vaporized tissue.32–35 None of the studies reviewed involved treatment within the oral cavity. A review of the literature reveals no cases of any dental personnel becoming ill as a result of inhaling laser plume. In any case, contaminants generated by lasers can and should be controlled by ventilation, safe work practices, and personal protective equipment.
Laser Techniques and Procedures
The basis of any laser technique starts with knowing the ins and outs of the laser system to maximize its use and prevent complications. Laser systems are equipped with an operator’s manual, detailing the safety features. The operator and assistants should thoroughly review the manual before using the laser and should strictly adhere to the indications. No health care personnel should attempt to use a laser on a patient without proper training (see Chapter 16).
The most frequent injuries resulting from the use of lasers are caused by laser energy emitted beyond the working area and striking the surrounding soft tissues. This typically occurs when a specimen is horizontally transected, because the laser energy may redirect off a reflective metal surface being used as an oral retractor. These complications usually result in minimal or no injury, but a patient’s initial response to the stimulus may be interpreted as pain. This can easily be avoided by obstructing the distant tissues with moist gauze or a tongue blade and by using matte-finished, nonreflective instrumentation.3
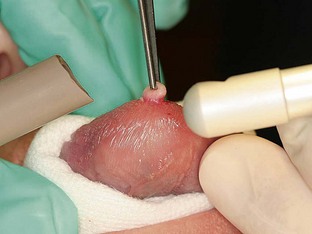
Wavelength-specific eyewear is mandatory, protecting the user from misdirected or reflected laser energy. During laser use, high-speed suction or evacuator and high-filtration masks should be worn to prevent illness from inhalation of the plume released at the site of energy-tissue interaction. To prevent ignition with flammable gases, nitrous oxide and oxygen must be temporarily discontinued during laser use to prevent serious harm to the patient. Because CO2 laser energy is well absorbed by hydroxyapatite, a major component of tooth enamel, significant amounts of laser energy absorbed by the teeth can cause etching and pitting, weakening enamel36 and increasing pulpal temperature.37 When in proximity to a CO2 laser, the teeth can be protected with moistened gauze or a fabricated tooth guard to absorb errant energy from the laser (Figure 6-1).
It is important to understand that one or more techniques may be necessary for any given clinical scenario, depending on three surgeon-controlled laser parameters: energy, time, and spot size.3 These parameters equate at the focal point of the laser energy emitted from any given laser handpiece. Altering the distance of the handpiece from the tissue serves to focus and defocus the laser beam’s focal point, therefore altering the effect of the laser on the target tissue. A laser in focus will excise, incise, ablate, or coagulate with the most efficiency. When the laser is out of focus, there will be less efficiency in ablation/incision/excision and more efficiency in coagulation.
Incision/Excision Techniques and Procedures
Focused mode is when the focal point of laser energy makes contact with tissue, maximizing the power per unit to a pinpointed area. Using the CO2 laser in a focused mode allows for increased depth, yet produces an incision thinner than a scalpel, functioning as a “light” scalpel. The characteristics of the CO2 laser make it ideal for most intraoral procedures traditionally performed with a scalpel, such as incision and excision biopsy, lesion removal, and flap elevation.3,7,38
Biopsy Procedure
Every patient requires a screening for cancer, which is accomplished by performing a thorough oral, head, and neck examination. Oral examinations can be made more efficient by inspecting high-risk sites, where 90% of oral squamous cell cancers arise: floor of the mouth, ventrolateral aspect of the tongue, and the soft palate complex.39 Many screening and detection aids can be used as adjunctive tools to identify precancerous and cancerous tissues early and before they become visible to the naked eye, expediting diagnosis and improving the patient’s prognosis. Oh and Laskin40 noted accentuation of some lesions when patients used acetic rinse, but there was no significant improvement in detection. Also, examination with the chemiluminescent ViziLite system produced reflections that made visualization more difficult.
When cellular changes are detected, the downfall of all adjunctive techniques is that a surgical biopsy procedure remains necessary to obtain a diagnosis.41–43 Biopsy is the process of removing a sample of tissue from a patient for diagnostic examination. The procedure is required to determine the underlying process within the tissues that is causing changes in their clinical appearance. The diagnosis obtained ranges from “normalcy” to an inflammatory process, and “systemic disease” to a benign or malignant neoplasm. Ultimately, an accurate diagnosis guides the clinician in determining the necessary treatment. The five intraoral biopsy techniques are aspiration biopsy, cytological biopsy, brush biopsy, excision biopsy, and incision biopsy.
The brush biopsy technique has improved the sensitivity (92.3%) and specificity (94.3%) for detection of oral squamous cell carcinoma or dysplasia when tested on visually identified lesions.44–46 Note that brush biopsies in particular should be used only as a screening tool, and atypical cell identification or positive results from such biopsies require an additional step implementing a surgical procedure to confirm a diagnosis.47
Excision Technique
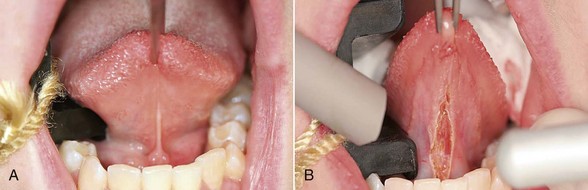
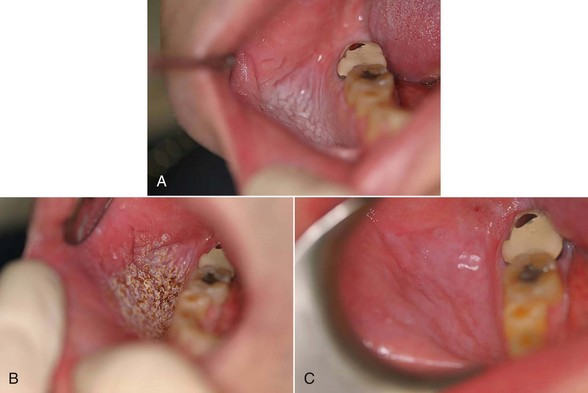
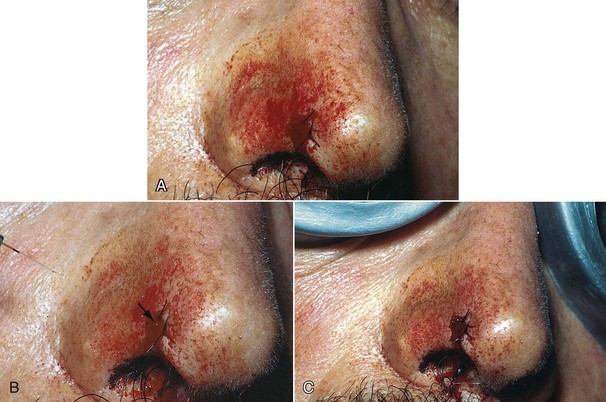
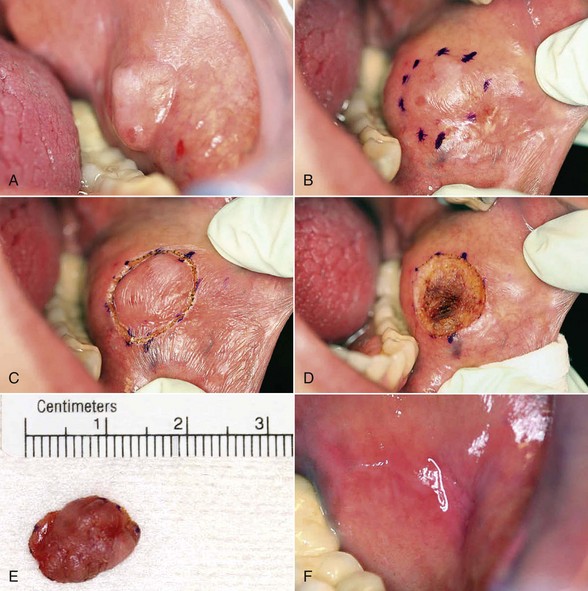
Excision technique requires the removal of the entire lesion with at least 2 to 3 mm of peripheral margin (Figure 6-5, A-C). This technique is preferred for oral lesions 1 cm or less and for minor, solid, and exophytic lesions. Localized discrete lesions such as fibroma, papilloma, mucocele, and pyogenic granuloma are most often excised with a laser.
When surgeons perform their first laser biopsy, they should take slightly wider margins than they would take with a blade, to decrease the possibility of thermal necrosis (a beginner’s mistake in biopsy technique) at the incision margins.38
Documentation
Digital photography is invaluable in dental practice today. A clinical photograph should be captured before administering anesthetic to preclude capturing any distortion in the involved tissues. It is vital to document all aspects of a patient’s treatment, including presurgical and postsurgical photographs, surgical margins, surgical defect(s), and the biopsy specimen (
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses