CHAPTER 6. Major infections of the mouth, jaws and perioral tissues
Syphilitic, actinomycotic and tuberculous osteomyelitis of the jaws are recognised entities, but largely of historical interest.
ACUTE OSTEOMYELITIS OF THE JAWS → Summaries pp. 66, 190, 448
Osteomyelitis of the jaws is mainly a disease of adults with several potential sources of infection (Box 6.1).
Box 6.1
Acute osteomyelitis of the jaws: potential sources of infection
• Periapical infection
• A periodontal pocket involved in a fracture
• Acute necrotising gingivitis or pericoronitis (even more rarely)
• Penetrating, contaminated injuries (open fractures or gunshot wounds)
Many patients have a predisposing cause and the most important are summarised in Box 6.2. Rare causes include osteopetrosis (Ch. 10). The effect of immunodeficiency is variable and acute osteomyelitis of the jaw is uncommon in HIV infection.
Box 6.2
Important predisposing conditions for osteomyelitis
Local damage to or disease of the jaws
• Fractures including gunshot wounds
• Radiation damage
• Causes of osteosclerosis
Paget’s disease
Late stages of fibro-osseous lesions
Osteopetrosis
Impaired immune defences
• Acute leukaemia
• Poorly controlled diabetes mellitus
• Sickle cell anaemia
• Chronic alcoholism or malnutrition
Clinical features
Most patients with osteomyelitis are adult males with infection of the mandible. Osteomyelitis of the maxilla is a rare disease of neonates or infants after either birth injuries or uncontrolled middle ear infection.
Early complaints are severe, throbbing, deep-seated pain and swelling with external swelling due to inflammatory oedema. Later, distension of the periosteum with pus and, finally, subperiosteal bone formation cause the swelling to become firm. The overlying gingiva is red, swollen and tender.
Associated teeth are tender. They may become loose and pus may exude from an open socket or gingival margins. Muscle oedema causes difficulty in opening the mouth and swallowing. Regional lymph nodes are enlarged and tender and anaesthesia or paraesthesia of the lower lip is characteristic.
Frequently, the patient remains surprisingly well but, in the acute phase, there may be fever and leucocytosis. A severely ill or very pale patient suggests underlying disease which requires investigation.
Radiographic changes do not appear until after at least 10 days. Loss of trabecular pattern and areas of radiolucency indicate bone destruction. These areas have ill-defined margins and have a fluffy or moth-eaten appearance (Fig. 6.1). Areas of dead bone appear as relatively dense areas which become more sharply defined as they are progressively separated as sequestra. Later, in young persons particularly, subperiosteal new bone formation causes a buccal swelling and appears as a thin, curved strip of new bone below the lower border of the jaw in lateral radiographs.
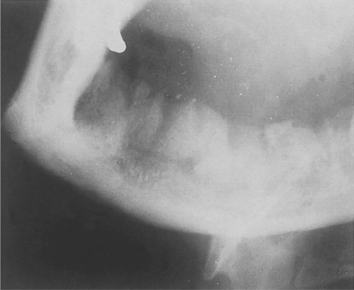 |
| Fig. 6.1
Osteomyelitis of the mandible following dental extractions. The outlines of the extraction sockets can be seen, together with dense sequestra of bone lying in a poorly circumscribed radiolucency.
|
Pathology
Oral bacteria, particularly anaerobes such as Bacteroides, Porphyromonas or Prevotella species are important causes, but the infection is often mixed. Staphylococci may be responsible when they enter from the skin via an open fracture.
The mandible has a relatively limited blood supply and dense bone with thick cortical plates. Infection causes acute inflammation in the medullary soft tissues and inflammatory exudate spreads infection through the marrow spaces. It also compresses blood vessels confined within the rigid boundaries of the vascular canals. Thrombosis and obstruction then lead to further bone necrosis. Dead bone is recognisable microscopically by lacunae empty of osteocytes but filled with neutrophils and colonies of bacteria which proliferate in the dead tissue (Fig. 6.2).
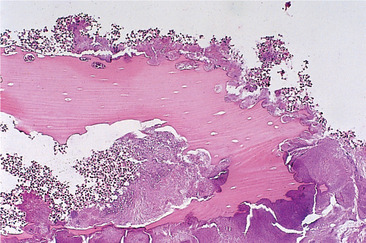 |
| Fig. 6.2
High-power view of a sequestrum showing non-vital bone (the osteocyte lacunae are empty) and eroded outline with superficial lacunae produced by osteoclastic resorption and a dense surface growth of bacteria.
|
Pus, formed by liquefaction of necrotic soft tissue and inflammatory cells, is forced along the medulla and eventually reaches the subperiosteal region by resorption of bone. Distension of the periosteum by pus stimulates subperiosteal bone formation, but perforation of the periosteum by pus and formation of sinuses on the skin or oral mucosa are rarely seen now.
At the boundaries between infected and healthy tissue, osteoclasts resorb the periphery of the dead bone which eventually becomes separated as a sequestrum (Fig. 6.3). Once infection starts to localise, new bone forms around it, particularly subperiosteally.
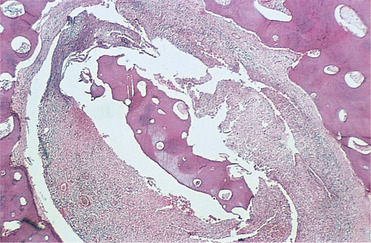 |
| Fig. 6.3
Late-stage chronic osteomyelitis. A sequestrum trapped in a cavity within the bone. It is surrounded by fibrous tissue containing an infiltrate of inflammatory cells. Surgical intervention is needed to remove an infected sequestrum such as this.
|
Where bone has died and been removed, healing is by granulation with formation of coarse fibrous bone in the proliferating connective tissue. After resolution, woven bone is gradually replaced by compact bone and remodelled to restore normal morphology.
Management
The main requirements are summarised in Box 6.3.
Box 6.3
Summary of management of osteomyelitis
Essential measures
• Bacterial sampling and culture
• Vigorous (empirical) antibiotic treatment
• Drainage
• Analgesics
• Give specific antibiotics based on culture and sensitivities
• Debridement
• Remove source of infection, if possible
Adjunctive treatment
• Sequestrectomy
• Decortication if necessary
• Hyperbaric oxygen*
• Resection and reconstruction for extensive bone destruction
* Mainly of value for osteoradionecrosis and, possibly, anaerobic infections.
Bacteriological diagnosis
A specimen of pus or a swab from the depths of the lesion must first be taken for culture and sensitivity testing.
Antimicrobial treatment
Immediately a specimen has been obtained, vigorous antibiotic treatment should be started. Initially, penicillin, 600–1200 mg daily can be given by injection (if the patient is not allergic), with metronidazole 200–400 mg 8-hourly. Clindamycin penetrates avascular tissue better and is frequently effective. The regimen is adjusted later in the light of the bacteriological findings.
Debridement
Removal of foreign or necrotic material and immobilisation of any fracture are necessary if there has been a gunshot wound or other contaminating injury.
Drainage
Pressure should be relieved by tooth extraction, bur holes or decortication, as necessary, and exudate drained into the mouth or externally.
Removal of sequestra
Dead bone should not be forcibly separated and vigorous curetting is inadvisable but, in the late stages, a loosened sequestrum may have to be removed. Teeth should be extracted only if loosened by tissue destruction.
Adjunctive treatment
Decortication or hyperbaric oxygen therapy, or both, may be attempted, particularly in radiation-associated osteomyelitis, though the effectiveness of hyperbaric oxygen is unproven.
Complications
Anaesthesia of the lower lip usually recovers with elimination of the infection. Rare complications include pathological fracture caused by extensive bone destruction, chronic osteomyelitis after inadequate treatment, cellulitis due to spread of exceptionally virulent bacteria or septicaemia in an immunodeficient patient.
Key features of acute osteomyelitis of the jaws are summarised in Box 6.4.
Box 6.4
Acute osteomyelitis of the jaws: key features
• Mandible mainly affected, usually in adult males
• Infection of dental origin – anaerobes are important
• Pain and swelling of jaw
• Teeth in the area are tender: gingivae are red and swollen
• Sometimes paraesthesia of the lip
• Minimal systemic upset
• After about 10 days, radiographs show moth-eaten pattern of bone destruction
• Good response to prompt antibiotic treatment and debridement
CHRONIC OSTEOMYELITIS → Summaries pp. 66, 190, 448
Rarely, inadequately treated acute osteomyelitis may lead to chronic suppurative osteomyelitis, which may also be a complication of irradiation (Fig. 6.4). Persistent low-grade infection is associated with bone destruction and granulation tissue formation, but little suppuration.
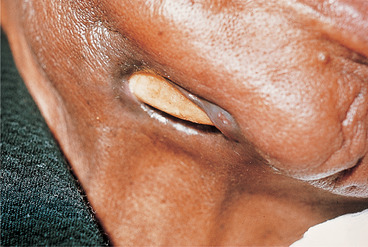 |
| Fig. 6.4
Chronic osteomyelitis secondary to radiotherapy. Part of the necrotic portion of the mandible is visible, having ulcerated through the skin.
|
Chronic osteomyelitis more frequently arises de novo as a result of infection by weakly virulent bacteria or in avascular bone.
Chronic osteomyelitis is commoner than acute osteomyelitis. Like the acute condition, there are usually predisposing factors such as those in Box 6.2. However, local bone sclerosis is a much more likely predisposing factor for chronic osteomyelitis than acute.
Clinically, the picture is often dominated by persistent ache or pain with exposed bone and radiographic changes rather than florid infection. Radiographic appearances are variable but sometimes distinctive (Fig. 6.5). Sequestra will usually separate spontaneously or may occasionally reincorporate into healing bone after effective antibiotic treatment. Chronic osteomyelitis must be treated aggressively. Infection may spread widely through abnormal bone or in a debilitated host (Fig. 6.6). Low-virulence organisms or repeated inadequate antibiotic treatment may lead to longstanding osteomyelitis with extensive sclerosis of the remaining viable bone, in so-called diffuse sclerosing osteomyelitis (Box 6.5).
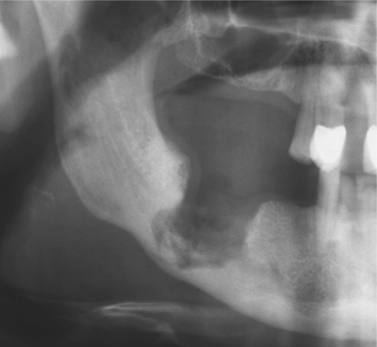 |
| Fig. 6.5
Chronic osteomyelitis. The extent of destruction is much more readily apparent than in acute osteomyelitis. Note the sequestra lying close to the lower border and the peripheral sclerosis. Subperiosteal new bone formation is evident below the lower border.
|
 |
| Fig. 6.6
Sequestration of the entire mandible following chronic osteomyelitis of odontogenic origin.
|
Box 6.5
Diffuse sclerosing osteomyelitis: key features
• Adults
• No sex predilection
• Sclerosis round site of periapical or periodontal chronic inflammation
• Persistent ache or pain but no swelling
• Radiographically resembles but is distinct from florid cemento-osseous dysplasia
Pathology
• Bone sclerosis and remodelling
• Scanty marrow spaces and little or no inflammatory infiltrate, though adjacent to areas of inflammation
Treatment
• Elimination of originating source of inflammation, but sclerotic areas remain radiographically
CHRONIC LOW-GRADE OSTEOMYELITIS AND OSTEITIS → Summaries pp. 173, 190
In some cases, a focus of osteomyelitis is so small or caused by such low-virulence organisms that the clinical presentation is dominated by the local bone reaction to the infection rather than the infection itself. Suppuration and infiltration of marrow spaces by inflammatory cells are absent and bacteria are not readily cultivable. Diffuse sclerosing osteomyelitis (see Box 6.5) was noted above. Other examples of such conditions are focal sclerosing osteomyelitis and proliferative periostitis.
Focal sclerosing osteomyelitis (Box 6.6) is characterised by sclerosis of the surrounding bone and is commoner in the young and in very longstanding infection.
Box 6.6
Focal sclerosing osteomyelitis: key features
• Bony reaction to low-grade periapical infection or unusually strong host defensive response
• Children and young adults affected
• Premolar or molar region of mandible affected
• Bone sclerosis associated with a non-vital or pulpitic tooth
• Localised but uniform radiodensity related to tooth with widened periodontal ligament space or periapical area
• No expansion of the jaw
Pathology
• Dense sclerotic bone with scanty connective tissue or inflammatory cells
Treatment
• Elimination of the source of inflammation by extraction or endodontic treatment
Chronic osteomyelitis in children and adolescents is unusual, but often elicits florid proliferative periostitis as a healing response (Box 6.7). Layers of periosteal new bone formation may expand the cortex by up to a centimetre or more in thickness. Vague pain and prominent bony enlargement are the main features and computerised tomography is useful to identify a small focus of chronic infection or sequestration in the expanded bone.
Box 6.7
Proliferative periostitis (‘Garrè’s osteomyelitis’*): key features
• Adolescents mainly affected
• Usually associated with periapical but sometimes other inflammatory foci
• Periosteal reaction affecting lower border of mandible causing ‘onion skin’ thickening and swelling of bone
Pathology
• Parallel layers of highly cellular woven bone interspersed with scantily inflamed connective tissue
• Small sequestra if present
Treatment
• Eliminate focus of infection
• Bone gradually remodels after 6 to 12 months
*Garrè’s osteomyelitis is a misnomer. In his original description he made no mention of proliferative changes in the lesion, X-rays had not then been invented and he provided no histological back-up.
Sclerosing osteitis is a term given to a localised area of sclerosis without evidence of infection. These are probably a reaction to inflammation rather than infection and are frequently seen around the roots of non-vital teeth (Fig. 6.7) resembling lesions of cemento-osseous dysplasia. No treatment is required other than to remove the causative tooth.
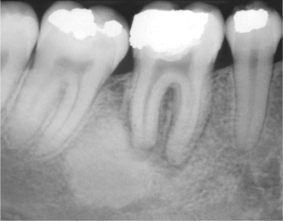 |
| Fig. 6.7
Sclerosing osteitis, a focal zone of sclerosis associated with periapical inflammation from a non-vital lower first molar.
Courtesy of Mr EJ Whaites.
|
BISPHOSPHONATE-INDUCED OSTEONECROSIS
Bisphosphonates inhibit bone resorption by suppressing osteoclast activity and are frequently used to lessen bone destruction by metastases to bone, particularly from multiple myeloma, breast and prostate carcinoma. This is highly effective, constraining growth of metastases and inhibiting pathological fractures and their consequences, particularly nerve injury from spinal collapse.
However, treatment with high-potency bisphosphonates such as alendronate, pamidronate and zoledronate, especially when administered long term and in high doses intravenously, can lead to osteonecrosis. 1 Occasional cases have also been reported following oral administration. Osteonecrosis affects primarily the jaws, usually the mandible.
The mechanisms of necrosis are unclear, but bisphosphonates reduce vascularity as well as inhibiting oseoclasts. It is not known whether the bone becomes necrotic before infection is introduced or whether necrosis results from infection. Typical presenting lesions are either non-healing extraction sockets or exposed bone; they are refractory to conservative treatment and antibiotic therapy. In many patients, dental surgery, particularly extractions, within the previous year appeared to be the precipitating factor. A striking presentation is also painless exposed bone; some patients may develop no acute symptoms or infection for prolonged periods.
Once infection is introduced, the condition develops into acute or chronic osteomyelitis depending on the virulence of the organism and resistance of the patient. The drugs cause reduced bone turnover so that sequestra of necrotic bone separate very slowly and large areas of exposed bone result (Figs 6.8 and 6.9). Later complications can include oroantral and cutaneous fistulas with suppuration. Surgical treatment is often not possible; the whole bone is involved.
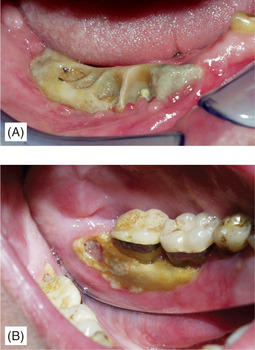 |
| Fig. 6.8
Exposed necrotic bone in bisphosphonate-induced osteonecrosis, (A) following extraction and (B) apparently spontaneous. Despite exposure of large areas of necrotic bone there is no overt infection in these early lesions.
Courtesy Dr J Fantasia. Published in Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endontology, Vol 102, Ruggiero SL et al, Bisphosphonate-related osteonecrosis of the jaw, pp 433–41, © Elsevier, 2006.
|
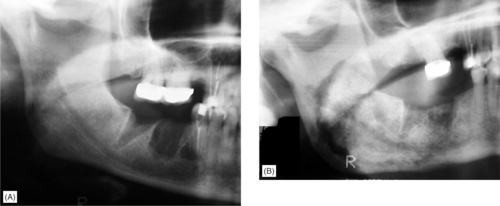 |
| Fig. 6.9
Bisphosphonate-induced osteonecrosis. Non-healing extraction sockets in a breast cancer patient receiving bisphosphonate treatment. (A) There are minimal changes initially but 12 months later, (B) there is a large sequestrum still incompletely separated extending from the premolar region, just above the lower border cortex and involving most of the ramus.
Courtesy Dr J Fantasia. Published in Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endontology, Vol 102, Ruggiero SL et al, Bisphosphonate-related osteonecrosis of the jaw, pp 433–41, © Elsevier, 2006.
|
Bisphosphonate osteonecrosis is a recently recognised condition and treatment guidelines are not yet evidence-based. Discontinuation of the bisphosphonates does not appear to help because their effects last at least a year after administration and may be permanent.
First principles dictate that prevention of infection is paramount. Potential problems should be eliminated before bisphosphonate treatment. After administration of bisphosphonates, patients at risk must be identified by medical history and additional predisposing factors sought (Box 6.8). Caries and periodontitis must be controlled. Ideally, all surgical dentistry should be avoided for as long as possible after drug administration and there is a role for temporary restorations and interim endodontics. If extractions cannot be delayed, they are probably best followed by postoperative antibiotics and chlorhexidine rinses until the sockets are fully epithelialised. Unfortunately, these precautions are not always successful.
Box 6.8
Risk factors for bisphosphonate-induced osteonecrosis
• Intravenous high-dose bisphosphonate treatment usually for bone metastases or hypercalcaemia of malignancy
• Radiotherapy to head and neck
• Immunosuppression from chemotherapy or steroids
• Anaemia
• Dental surgery or sepsis, ill-fitting dentures and poor oral hygiene
• Female patients
• Elderly patients
• Smoking
When bone is exposed but symptoms are minimal, chlorhexidine mouth rinses reduce pain and the risk of infection. If there is infection or soft tissue swelling, aggressive and long-term antibiotic therapy based on the results of antibiotic culture and sensitivity is required. Sev/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


