Infection Control and Management of Hazardous Materials
Learning Objectives
1 Pronounce, define, and spell the Key Terms.
3 Explain the components of the OSHA Bloodborne Pathogens Standard.
4 Explain the difference between Universal Precautions and Standard Precautions.
5 Describe the indications for the hepatitis B vaccine.
6 Describe and demonstrate the proper disposal of sharps.
7 Describe and demonstrate the proper technique of handwashing.
8 Describe the appropriate use of alcohol-based hand rubs.
9 Explain the requirements for personal protective equipment.
10 Describe the necessary precautions when handling contaminated laundry.
11 Describe the types of dental waste and the management of each.
12 Identify the five components of the OSHA Hazard Communication Standard.
Key Terms
Alcohol-Based Hand Rubs
Bloodborne Pathogens Standard (BBP)
Centers for Disease Control and Prevention (CDC)
Chemical Label
Hazard Communication Standard
Material Safety Data Sheets (MSDS)
Occupational Exposure
Occupational Safety and Health Administration (OSHA)
Percutaneous
Permucosal
Personal Protective Equipment (PPE)
Regulated Waste
Sharps
Standard Precautions
Universal Precautions
Roles and Responsibilities of the CDC and OSHA
The Centers for Disease Control and Prevention (CDC) and the Occupational Safety and Health Administration (OSHA) are federal agencies that have very important roles in infection control for dentistry.
The CDC is not a regulatory agency. Its role is to issue specific recommendations based on sound scientific evidence on health-related matters. Although not law, the CDC Guidelines for Infection Control in Dental Health Care Settings are now the standard of care (Figure 6-1 and Box 6-1).
OSHA is a regulatory agency. Its role is to issue specific regulations, also called standards, to protect the health of employees in the United States. Failure to comply with OSHA requirements can have serious consequences, including heavy fines.
As a dental assistant, it is important to follow all of the guidelines and recommendations.
This chapter discusses the basic concepts and step-by-step procedures you will need to prevent the transmission of disease and to safely manage hazardous material in the dental office.
OSHA’s Bloodborne Pathogens Standard
OSHA’s Bloodborne Pathogens Standard (BBP) is the most important infection control law in dentistry. It is designed to protect employees against occupational exposure to bloodborne, disease-causing organisms, such as hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV).
The BBP requires employers to protect their employees from exposure to blood and other potentially infectious material (OPIM) in the workplace and to provide proper care to the employee if an exposure should occur. The standard applies to any type of facility in which employees might be exposed to blood and other body fluids, including dental and medical offices, hospitals, funeral homes, emergency medical services, and nursing homes.
OSHA requires that a copy of the BBP Standard be present in every dental office and clinic. A copy of the OSHA BBP Standard may be obtained by visiting http://www.osha.gov/SLTC/bloodbornepathogens/index/html.
Exposure Control Plan
Each dental office must have a written exposure control plan that clearly describes how that office complies with the BBP Standard. The exposure control plan must be reviewed and updated at least annually. A copy must be accessible to all employees (Box 6-2).
Standard and Universal Precautions
The term Universal Precautions is still referred to in the OSHA BBP Standard. Universal Precautions is based on the concept that all human blood and certain body fluids (including saliva) are to be treated as if known to be infected with the bloodborne disease hepatitis B, hepatitis C, or HIV infection. The rationale for this concept is that it is not possible to identify those individuals who are infectious, so Universal Precautions were to be used for all healthcare personnel and their patients.
The CDC expanded the concept and changed the term to Standard Precautions. Standard Precautions apply not just to contact with blood, but also to (1) all body fluids, secretions, and excretions (except sweat), regardless of whether they contain blood; (2) nonintact skin; and (3) mucous membranes. Saliva has always been considered a potentially infectious material in dental infection control; therefore there is no difference in clinical dental practice between Universal Precautions and Standard Precautions. Standard Precautions apply to contact with:
Categories of Employees
The OSHA BBP Standard requires employers to categorize tasks and procedures during which an employee might have an occupational exposure (Table 6-1).
TABLE 6-1
Occupational Exposure Determination
| Category | Definition | Example |
| I | Routinely exposed to blood, saliva, or both | Dentist, dental hygienist, dental assistant, sterilization assistant, dental laboratory technician |
| II | May on occasion be exposed to blood, saliva, or both | Receptionist or office manager who may on occasion clean a treatment room, handle instruments or impressions, etc. |
| III | Never exposed to blood, saliva, or both | Financial manager, insurance clerk, or computer operator may belong in this category |
The BBP defines an occupational exposure as “any reasonably anticipated skin, eye, [or] mucous membrane contact, or percutaneous injury, with blood or any other potentially infectious materials.” Percutaneous (through the skin, such as needle sticks, cuts, and human bites) and permucosal (contact with mucous membranes, such as the eyes or mouth) exposures to blood, saliva, and other body fluids pose the greatest risk for transmission of HIV, HBV, and HCV.
Employee Training
The BBP Standard requires the dentist and/or employer to provide training in infection control and safety issues to all personnel who may come in contact with blood, saliva, or contaminated instruments or surfaces. The employer must keep records of all training sessions. The record of each training session must include the date of the session, the name of the presenter, the topic, and the names of all employees who attended (Box 6-3).
Hepatitis B Immunization
The BBP Standard requires the dentist and/or employer to offer the HBV vaccination series to all employees whose jobs include category I and II tasks. The vaccine must be offered within 10 days of assignment to a category I or II job. To document compliance, the dentist/employer must obtain proof from the physician who administered the vaccination to the employee.
The employee has the right to refuse the HBV vaccine for any reason. The employee is then required to sign an informed refusal form that is kept on file in the dental office. Even if the employee originally signed the refusal form, the employee always has the right to reverse the decision and receive the vaccine at a later date at no charge.
Need for an HBV Booster
The CDC does not recommend routine booster doses of the HBV vaccine, nor does it recommend routine blood testing to monitor the HBV antibody level in individuals who have already had the vaccine. This is assuming that the individual was tested after receiving the vaccine and was known to have initially developed antibodies. An exception is the immunized individual who has a documented exposure incident and for whom the attending physician orders a booster dose.
Employee Medical Records
The dentist/employer must keep a confidential medical record for each employee. The employer must store these records in a locked file for the duration of employment plus 30 years (Box 6-4).
Managing Contaminated Sharps
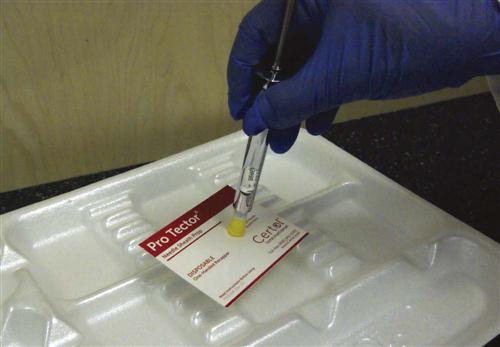
Contaminated needles and other disposable sharps, such as scalpel blades, orthodontic wires, and broken glass, must be placed into a sharps container. The sharps container must be puncture resistant, closable, leakproof, and color-coded or labeled with the biohazard symbol (Figure 6-2).
Sharps containers must be located as close as possible to the place of immediate disposal. Do not cut, bend, or break the needles before disposal. Never attempt to remove a needle from a disposable-type device.
Preventing Needle Sticks
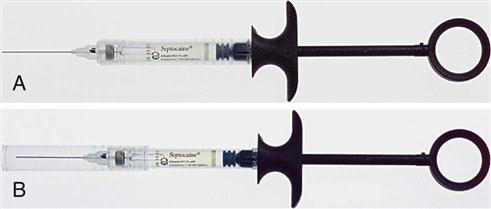
Some needles on the market have safety guards to prevent accidental needle sticks (Figure 6-3). Do not bend or break needles before disposal. Always use the single-handed scoop technique or some type of safety device (Figure 6-4).
Postexposure Management
Despite efforts to prevent occupational exposure incidents, accidents happen. Therefore, before an accident occurs, the BBP Standard requires the employer to have a written plan. This plan explains exactly what steps the employee must follow after the exposure incident occurs and the type of medical follow-up that will be provided to the employee at no charge (Box 6-5).
The employer must provide training to employees on the proper response to an exposure incident. Procedure 6-1 reviews first-aid steps after an exposure incident.
Handwashing and Hand Care
Handwashing
You must wash your hands before you put on gloves (Procedure 6-2) and immediately after you remove gloves. Handwashing is also required if you inadvertently touch contaminated objects or surfaces while barehanded. You should always use liquid soap during handwashing. Bar soap should never be used because it may transmit contamination.
To minimize cross-contamination, it is preferable that treatment room sinks be equipped with “hands-free” faucets that are activated electronically or with foot pedals (Figure 6-5).
Alcohol-Based Hand Rubs
A new category of antiseptic products is now available on the market for hand hygiene. Waterless antiseptic agents are alcohol-based products that are available in gels, foams, or rinses (Figure 6-6). They do not require the use of water. The product is simply applied to the hands, which are then rubbed together to cover all surfaces.
These products are more effective at reducing microbial flora than plain soap, or even an antimicrobial hand wash. Concentrations of 60% to 95% are the most effective. In addition, these products are actually good for your skin. They contain emollients that reduce the incidence of chapping, irritation, and drying of the skin.
Alcohol-based hand rubs are not indicated if your hands are visibly soiled or are contaminated with organic matter, such as blood or saliva. In this case, you would need to wash your hands first with soap and water, and then follow with the alcohol-based product (Procedure 6-3).
Hand Care Recommendations
Healthy skin is better able to withstand the damaging effects of repeated washing and of wearing gloves. It is important to dry your hands well before donning gloves.
Dental personnel with open sores or weeping dermatitis must avoid activities involving direct patient contact and handling contaminated instruments or equipment until the condition on the hands is healed.
Because rings and long fingernails can harbor pathogens, nails should be kept short and well manicured. Rings, long nails, and artificial nails are likely to puncture examination gloves. In addition, microorganisms can enter the body through any break in the skin. CDC guidelines recommend that rings, fingernail polish, and artificial nails should not be worn at work.
Personal Protective Equipment
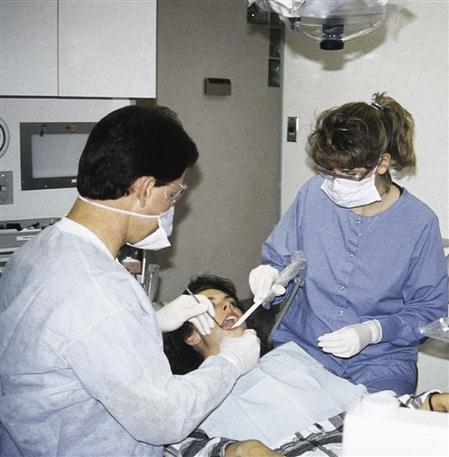
OSHA’s BBP Standard requires the employer to provide employees with appropriate personal protective equipment (PPE) (Figure 6-7) without charge to the employee. Examples of PPE include protective clothing, surgical masks, face shields, protective eyewear, disposable patient treatment gloves, and heavy-duty utility gloves.
Because dental assistants are likely to come in contact with blood and saliva, you must wear PPE whenever you are performing tasks that could produce contact with body fluids (Procedures 6-4 and 6-5).
You must also wear appropriate PPE when you perform other clinical activities that require handling items contaminated with patient secretions. Examples include processing dental radiographs and handling laboratory cases, dentures, and other prosthetic appliances or contaminated equipment and surfaces.
Protective Clothing
The purpose of protective clothing is to protect the skin and underclothing from exposure to saliva, blood, aerosol, and other contaminated materials. Types of protective clothing can include smocks, pants, skirts, laboratory coats, surgical scrubs (hospital operating room clothing), scrub (surgical) hats, and shoe covers. Technically, clinic shoes and hosiery also are part of PPE.
The decision as to the type of protective clothing you should wear is based on the degree of anticipated exposure to infectious materials. For example, assisting with the high-speed handpiece during a cavity preparation carries a high risk of exposure to contaminated aerosol. Charting during an oral examination, on the other hand, carries a lower risk of exposure because it does not involve use of the handpiece or air-water syringe, which creates contaminated aerosol (Figure 6-8).
Protective Clothing Requirements
Protective clothing should be made of fluid-resistant material. Cotton, cotton/polyester, or disposable jackets or gowns usually are satisfactory for routine dental procedures.
To minimize the amount of uncovered skin, clothing should have long sleeves and a high neckline.
The design of the sleeve should allow the cuff to be tucked inside the band of the glove.
During high-risk procedures, protective clothing must cove/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses