Finishing, Polishing, and Cleansing Materials
Objectives
After reading this chapter, the student should be able to:
Abrasion
< ?mpslid E1?>< ?mpslid S2?>
< ?mpslid E2?>< ?mpslid S3?>
3. Define abrasion and contrast abrasive tools or slurries with cutting instruments.
< ?mpslid E3?>< ?mpslid S4?>
< ?mpslid E4?>< ?mpslid S5?>
5. Describe surface roughness and gloss.
< ?mpslid E5?>< ?mpslid S6?>
6. Give two principles of finishing and polishing techniques.
< ?mpslid E6?>< ?mpslid S7?>
7. List two reasons why an abrasive should not be used in a dry condition.
< ?mpslid E7?>< ?mpslid S8?>
< ?mpslid E8?>
Prophylactic Pastes
1. Give two ideal functions of a dental prophylactic paste.
< ?mpslid E9?>< ?mpslid S10?>
2. List the major abrasives and therapeutic agents used in prophylactic pastes.
< ?mpslid E10?>< ?mpslid S11?>
3. Compare cleansing and abrasion of tooth structure by various products.
< ?mpslid E11?>< ?mpslid S12?>
< ?mpslid E12?>
Dentifrices
1. Give the primary function of a dentifrice.
< ?mpslid E13?>< ?mpslid S14?>
2. Recognize four desirable effects of toothbrushing.
< ?mpslid E14?>< ?mpslid S15?>
3. List four types of debris in order of increasing difficulty of removal from surfaces of teeth.
< ?mpslid E15?>< ?mpslid S16?>
4. Recognize the components in a dentifrice, and indicate their function.
< ?mpslid E16?>< ?mpslid S17?>
5. List several common abrasives used in dentifrices.
< ?mpslid E17?>< ?mpslid S18?>
< ?mpslid E18?>< ?mpslid S19?>
7. List four variables of a toothbrush that can influence abrasion caused by a dentifrice.
< ?mpslid E19?>< ?mpslid S20?>
8. List four guidelines to follow in recommendation of a dentifrice for a patient.
< ?mpslid E20?>
Denture Cleansers
1. List six requirements of an ideal denture cleanser.
< ?mpslid E21?>< ?mpslid S22?>
2. List three major types of denture cleansers, and identify the active ingredient in each.
< ?mpslid E22?>< ?mpslid S23?>
3. Give the disadvantages of each type of denture cleanser.
< ?mpslid E23?>< ?mpslid S24?>
4. Describe effective techniques for cleaning dentures, including those with soft liners.
< ?mpslid E24?>< ?mpslid S25?>
< ?mpslid E25?>
Whitening
1. Indicate types of stains for which in-office whitening techniques may be effective.
< ?mpslid E26?>< ?mpslid S27?>
2. Compare the ingredients of in-office and home whitening agents.
< ?mpslid E27?>< ?mpslid S28?>
3. Indicate the effect of whitening agents on restorative materials.
< ?mpslid E28?>< ?mpslid S29?>
4. Give side effects reported for whitening agents.
< ?mpslid E29?>< ?mpslid S30?>
5. List three major methods of in-office whitening.
< ?mpslid E30?>< ?mpslid S31?>
6. Describe an in-office whitening gel technique.
< ?mpslid E31?>< ?mpslid S32?>
7. Describe a home whitening technique.
< ?mpslid E32?>< ?mpslid S33?>
8. Describe universal whitening guidelines and additional guidelines for in-office whitening gels.
< ?mpslid E33?>
Key Terms
Binder
Cleansing abrasive
Coating
Finishing abrasive
Gloss
Humectant
Indirect technique
Noble metal
Perlite
Polishing abrasive
Rouge
Silicon carbide
Soft liner
Finishing and polishing techniques are meant to remove excess material and smooth roughened surfaces. A rough surface on a restoration may be uncomfortable and make oral hygiene difficult, because food debris and plaque can cling to it easily. When a restoration is located in proximity to the gingiva, surface roughness can cause painful irritation and eventual recession of the soft tissue. Roughness of metallic restorative materials is responsible for accelerating corrosion. The finishing and polishing of restorative dental materials are important steps in the fabrication of clinically successful restorations.
Cleansing techniques are meant to remove food and other debris from a surface without damaging it. Polishing and cleaning are routine procedures for maintaining the health of the natural dentition. These procedures, however, can lead to roughened enamel surfaces by the use of excessively abrasive dentifrices at home or coarse prophylactic slurries at the dental office. Dentifrices and prophylactic pastes also can abrade some restorative materials during a cleansing procedure.
The materials used for finishing and polishing are primarily abrasives. Most cleansing materials are also abrasives, although many chemical cleansing agents for denture bases exist. An understanding of the properties of these materials and the process of abrasion can improve clinical usage of finishing, polishing, and cleansing materials.
Abrasion
Abrasion results when a hard, rough surface, such as a sandpaper disk, or hard, irregularly shaped particles, such as those present in an abrasive slurry, plow grooves into a softer material and cause material from such grooves to be removed from the surface. The action of an abrasive is essentially a cutting action. Abrasive tools or slurries, however, differ from dental cutting instruments in that the cutting edges or points of the abrasive are not arranged in any particular pattern. Each point or edge of an abrasive acts as an individual cutting blade and removes some material from the surface being abraded.
The process of abrasion is affected by the physical and mechanical properties of the material being abraded. Properties such as hardness, strength, ductility, and thermal conductivity are important. These properties are discussed later in this chapter with respect to the abrasion of individual restorative materials.
Rate
The rate of abrasion of a given material by a given abrasive is determined primarily by three factors: the size of the abrasive particle, the pressure of the abrasive against the material being abraded, and the speed at which the abrasive particle moves across the surface being abraded. All of these factors can be controlled clinically.
The size of an abrasive particle is an important factor in the rate at which the surface is abraded. Larger particles cause deeper scratches in the material and wear away the surface at a faster rate. The use of a coarse abrasive is indicated on a surface with many rough spots or large nodules. Finer abrasives are then used to remove the scratches caused by the coarse abrasive. New abrasive systems have particles that wear during use, which produces finer particles and an increasingly smooth finish.
A second important factor is the pressure of the abrasive against the surface being abraded. Heavy pressure applied by the abrasive causes deeper scratches and more rapid removal of material. However, heavy pressure also may cause the abrasive to fracture or to dislodge from the grinding wheel and thereby reduce cutting efficiency. Operator control of the abrasion process is lessened when excessive pressure is exerted because material is worn away too rapidly to keep the abrasion from occurring uniformly over the entire surface of the material. Judgment must be exercised in the amount of force applied to the dental handpiece or to the surface that is against a grinding wheel to avoid excessive pressure.
A third factor that controls the rate of abrasion is the speed at which the abrasive travels across the surface being abraded. The higher the speed, the greater is the frequency per unit of time the particle contacts the surface. Increasing the speed increases the rate of abrasion. In a clinical situation, it is easier to control speed rather than pressure to vary the rate of abrasion. Varying the speed has the additional advantage of using low pressure during maintenance of a high cutting efficiency.
Surface Roughness and Gloss
Surface roughness (Ra) is a measure of the irregularity of the finished and polished surface and is measured in micrometers (µm). A smooth surface (Ra less than 0.2 µm) is desirable to reduce retention of bacteria and to have a shiny appearance.
Gloss is a measure of the reflection of light from a surface. A totally nonreflective surface has zero gloss units (GU), and a perfect mirror will read 1000 GU at a measuring angle of 60 degrees. A gloss value of less than 10 is considered to be low in gloss, 10 to 70 is considered semigloss, and greater than 70 is considered high gloss. An ideal restorative material would have high gloss after polishing and would retain its gloss during function in the mouth.
Types of Abrasives
The three types of abrasives used in dentistry can be classified as finishing, polishing, and cleansing abrasives. Finishing abrasives are generally hard, coarse abrasives used primarily for development of desired contours of a restoration or tooth preparation and for removal of gross irregularities on the surface. Polishing abrasives have finer particle sizes and are generally less hard than abrasives that are used for finishing. The polishing abrasives are used to smooth surfaces roughened typically by finishing abrasives or wear particles encountered in the mouth. Cleansing abrasives are generally soft materials with small particle sizes and are intended to remove softer materials that adhere to enamel or restorative material substrates.
Dental abrasives are applied by means of a number of tools. The abrasive particles may be glued onto plastic or paper disks that can be attached to a dental handpiece or attached to strips for finishing of interproximal areas. Paper disks are preferable for finishing contoured surfaces because they are more flexible than plastic disks. The waterproof variety of paper disks is more durable. In the case of diamond rotary instruments, diamond chips are attached to steel wheels, disks, and cylinders. With grinding wheels and dental stones, the abrasive particles are bonded by a matrix material that is molded to form tools of desired sizes and shapes. The abrasive tools just described are used only for finishing.
Abrasives also may be mixed with water, glycerin, or some other medium to produce slurries or pastes. The use of glycerin as a medium prevents the change in consistency that occurs when water, which evaporates, is used to make a slurry. The slurry or paste then is rubbed over the surface of the material being abraded with a cloth or felt wheel, brush, or rubber cup. Abrasive slurries and pastes are used most commonly in dentistry for polishing and cleaning.
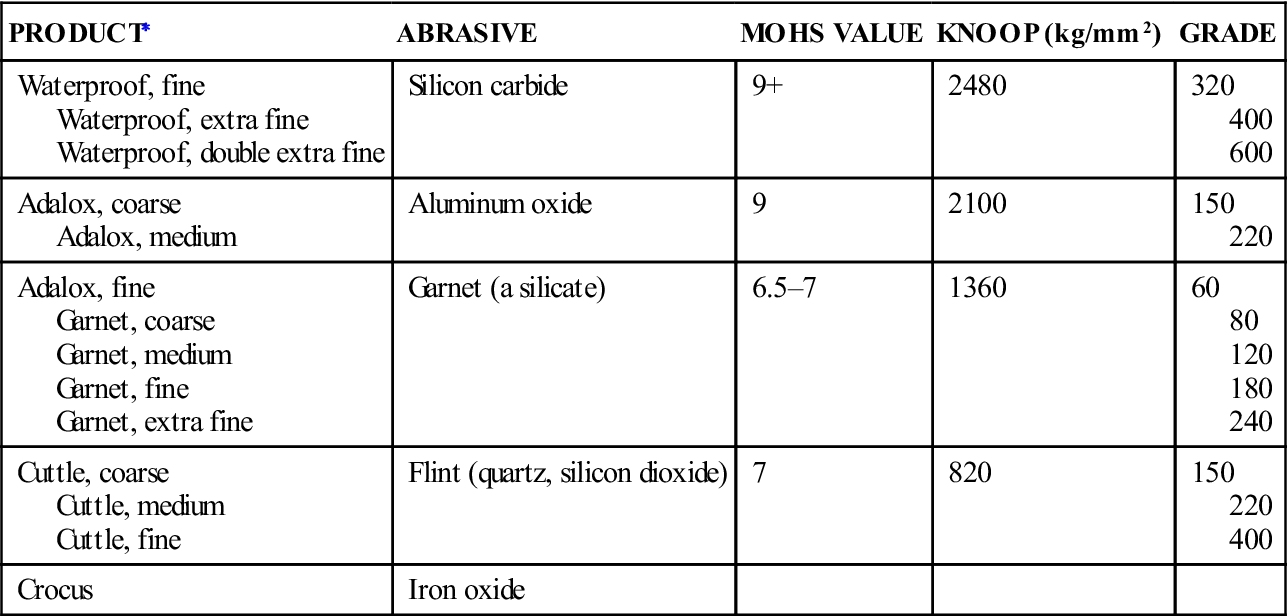
The following is a brief discussion of the abrasive agents commonly used for finishing. Values of hardness and grades of abrasives used on some commercial disks are listed in Table 6-1.
TABLE 6-1
Hardness and Grade of Various Types of Finishing Disks
< ?comst?>
| PRODUCT∗ | ABRASIVE | MOHS VALUE | KNOOP (kg/mm2) | GRADE |
| Waterproof, fine Waterproof, extra fine Waterproof, double extra fine |
Silicon carbide | 9+ | 2480 | 320 400 600 |
| Adalox, coarse Adalox, medium |
Aluminum oxide | 9 | 2100 | 150 220 |
| Adalox, fine Garnet, coarse Garnet, medium Garnet, fine Garnet, extra fine |
Garnet (a silicate) | 6.5–7 | 1360 | 60 80 120 180 240 |
| Cuttle, coarse Cuttle, medium Cuttle, fine |
Flint (quartz, silicon dioxide) | 7 | 820 | 150 220 400 |
| Crocus | Iron oxide |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
< ?comst1?>< ?comen1?>∗< ?comst1?>< ?comen1?>E.C. Moore Co., Inc., Dearborn, MI.
From Charbeneau GT: Unpublished data, University of Michigan School of Dentistry, Ann Arbor, MI.
Aluminum oxide (Al2O3) is an abrasive manufactured from an impure aluminum oxide (bauxite) and produced in various particle sizes. The particles are applied most commonly to paper or plastic disks in coarse, medium, and fine grits. The disks are reddish brown. Aluminum oxide powders (typically 27- and 50-µm particle sizes) are used in air-abrasion units.
Cuttle is an abrasive manufactured from the bones of fish, although this form is no longer used as a dental abrasive. Presently, cuttle is a trade name that refers to a fine grade of quartz (SiO2). The particles are applied to a paper disk in coarse, medium, and fine grits. The medium cuttle grit is similar in abrasive action to fine sand grit. Cuttle disks are beige.
Diamond is the hardest known substance. Diamond chips normally are impregnated in a binder to form diamond “stones” and disks. Disks, cups, and points with microfine diamonds (PoGo; DENTSPLY Caulk, Milford, Delaware) are available for polishing resin composite restorations to achieve a high gloss (Figure 6-1).
Garnet is an abrasive that is mined. In pure form, it is composed of oxides of aluminum, iron, and silicon. Garnet is available on paper or plastic disks in extra-coarse, coarse, medium, fine, and extra-fine grits, and is red.
Sand is a form of quartz (SiO2) used as an abrasive agent. It is available on plastic or paper disks in coarse, medium, and fine grits and is beige. Sand disks should not be used interchangeably with cuttle disks, although they are also of quartz, because the particle sizes of the coarse, medium, and fine grits are not the same for both abrasives.
Silicon carbide (SiC) is the second hardest of the dental abrasives and usually is applied to paper or plastic disks. The disks are available in fine, extra-fine, and double extra-fine grits and are black.
The abrasive agents commonly used in dentistry for polishing and cleansing follow. Calcite is a form of calcium carbonate (CaCO3). It is available in various grades as used in prophylactic pastes. Another physical form of calcium carbonate is chalk, which is used in dentifrices as a polishing agent.
Kieselguhr is a polishing agent and is composed of the siliceous remains of minute aquatic plants known as diatoms. The coarse form of kieselguhr is known as diatomaceous earth.
Pumice is a highly siliceous volcanic glass that when ground is useful as a polishing agent in prophylactic pastes and for finishing acrylic denture bases in the laboratory.
Rouge is a fine red powder composed of iron oxide (Fe2O3) that usually is used in cake form. It may be impregnated in paper or fabric known as crocus cloth. It is an excellent laboratory polishing agent for gold and other noble metal alloys.
Silex refers to siliceous materials such as quartz or tripoli, which are used as polishing abrasives in the mouth.
Tin oxide (SnO2) is a pure white powder used extensively as a final polishing agent for teeth and metallic restorations in the mouth. It is mixed with water, alcohol, or glycerin and used as a paste.
Tripoli is a polishing agent that originates from certain porous rocks found in North Africa. It often is confused with kieselguhr.
Zirconium silicate (ZrSiO4) is a hard abrasive that, in small particle sizes, is used as a polishing agent.
In addition to the abrasive agents already cited, several other abrasives are found in prophylactic pastes, including quartz, anatase (TiO2), feldspar, montmorillonite, aluminum hydroxide, kaolinite, and talc. Further information on prophylactic materials is presented later in this chapter.
The abrasives found in dentifrices include calcium carbonate, dibasic calcium phosphate dihydrate, anhydrous dibasic calcium phosphate, tricalcium phosphate, calcium pyrophosphate, sodium metaphosphate, hydrated alumina, and silica. These are mainly cleansing and polishing abrasives not meant to abrade enamel severely.
Finishing and Polishing Techniques
The finishing and polishing techniques for most restorative dental materials follow similar principles. Initial contouring and smoothing of the surface are done with a coarse abrasive or bur. Successively finer abrasives then remove the large scratches produced. The use of too fine an abrasive after a coarse one is time consuming and does not give a properly finished surface. A key to successful finishing and polishing is strict adherence to a recommended abrasive sequence.
With each successive change in abrasive, the area being finished and polished is rinsed to remove the previously used abrasive particles. One remaining particle of coarse abrasive can mar a well-polished surface. The abrasive tool or slurry must not be used in a dry condition. Dry polishing may reduce dramatically the efficiency of the abrasive and increase the danger of overheating the surface.
The abrasives chosen to finish and polish various restorative materials depend to a great extent on the properties of the particular restorative material. The discussion that follows involves consideration of the surface roughness that is caused by various abrasive agents, a recommended finishing and polishing sequence, and the precautions that should be taken in finishing and polishing some common restorative materials.
Amalgam
The average surface roughness produced by various methods of instrumentation on amalgam is listed in Table 6-2. A suggested abrasive sequence for finishing and polishing an occlusoproximal restoration is indicated therein.
TABLE 6-2
Average Surface Roughness of Dental Amalgam Produced by Various Methods of Instrumentation
< ?comst?>
| METHOD OF INSTRUMENTATION | ROUGHNESS (Ra, µm) |
| Carved | 4.6∗ |
| Carved and immediately smoothened (burnished) | 0.36∗ |
| Condensed against uncontoured matrix band | 0.61 |
| Rotating Finishing Instruments | |
| S.S. White green stone | 0.64–1.0 ∗ |
| Finishing bur | 0.46–0.64∗ |
| Waterproof (silicon carbide) fine | 0.58∗ |
| Rotating Polishing Instruments | |
| Robinson Soft Cup BrushWith extra-fine silex | 0.18∗ |
| With tin oxide | 0.10∗ |
| Interproximal Finishing Strips | |
| Moyco “Evenwe/> | |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses