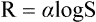Orofacial Pain
Pain Physiology
Common Terminologies Associated with Pain
1. Nociceptors: These are receptors that are sensitive to painful stimulus and are responsible for initiating the generation of pain.
2. Nociception: It is defined as a noxious stimulus or has potential to turn noxious over a period of time.
3. Allodynia: Pain that is produced by a stimulus that is not normally painful.
4. Hyperalgesia: Increased sensitivity to painful stimuli.
5. Hypoesthesia: Reduced sensation in response to stimulus.
6. Anesthesia: Absence of sensation in response to a stimulus.
7. Causalgia: Persistent burning pain caused by deafferentation of sensory innervation.
8. Neuralgia: It is the pain that is experienced in the tissues along the distribution of the nerve.
Properties of Pain
Weber and Fechner’s law
This law can be mathematically expressed as
where R = intensity of the reaction (i.e. the pain perceived),
S = the intensity of the stimulus.
According to this law, exponential increase in the intensity of stimulus does not cause exponential increase in pain perceived. Though the pain perceived increases with increase in stimulus; the pain experienced is only in terms of log of the intensity of the stimulus.
Nociceptors
Two general types of nociceptors are characterized by the neurons associated with them.
| Aδ fiber | C fiber |
| 1–6 μm diameter | 1.5 μm diameter |
| Myelinated | Non-myelinated |
| 5–30 m/s conduction velocity | 0.5–2 m/s conduction velocity |
| Carries the first pain that is experienced–sharp | Carries steady dull pain |
Sequelae of Pain
Muscular sequelae
Injury or disease causing pain results in spasm of skeletal muscle in the vicinity of the affected region. This is protective as it immobilizes the affected region and thereby puts it to forcible rest which is most essential for rapid healing. But this spasm also causes ischemia of the muscles which aggravates muscular pain.
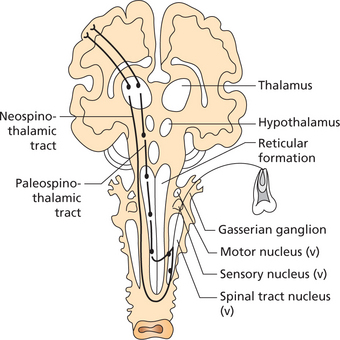
Dual Pain Pathways
Once these fibers (Aδ and C) enter the spinal cord, the pain signals take two pathways to the brain. They are the neospinothalamic tract and the paleospinothalamic tract (Figure 1).
Analgesia system or pain inhibitory pathway
The analgesia system comprises three major components, namely,
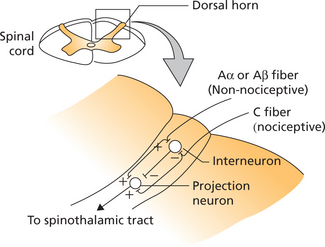
Gate Control Theory
There have been various modifications of the original gate control theory. A revised version of the gate control hypothesis proposes that the projection neuron of the spinothalamic pathway when activated results in sensation of pain (Figure 2).
Unlike pain, itching occurs in superficial tissues and not in deep structures like viscera.
Concept of Referred Pain
Characteristics of referred pain
1. Referred pain usually occurs within a single nerve root, passing from one branch to the other.
2. Referred pain in the trigeminal area rarely crosses the midline unless it originates at the midline.
3. Usually if the referred pain is felt outside the nerve that mediates the pain, it is generally felt cephalad to the nerve (upward and toward the head) and not caudally. However in severe pain the excitatory effects are felt caudal to the site of initiating input.
Classification of Orofacial Pain
The American Academy of Orofacial Pain has classified orofacial pain as follows:
Bell (1989) has classified orofacial pain as follows:
Clinical Assessment of Pain
The interview is followed by a complete oral and head and neck examination, appropriate chair side investigations, radiographic imaging and lab studies.
The location of pain and referral patterns can be identified by the patient using a pain diagram (Figure 3).
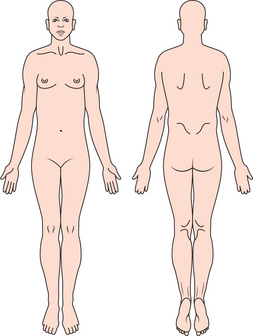
Figure 3 Illustration showing the pain diagram. Patient is encouraged to mark areas on the picture that represent the painful sites in the patient
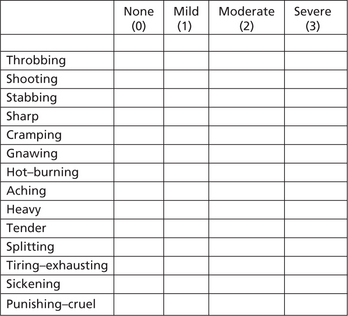
The pain quality or character can be expressed using the widely accepted McGill Pain Questionnaire (Figure 4). This questionnaire will help the patient to describe how exactly he/she feels about the pain by selecting an appropriate adjective from a list in the questionnaire.
The intensity of pain can be quantified using the visual analog scale (VAS). The VAS is a 10 cm long line with 0 marked on one end (represents no pain) and 10 at the other end (represents worst possible pain). The linear scale has markings from 0 to 10 at 1 cm intervals. The patient is encouraged to mark a point along this scale that correlates with the intensity of pain experienced. For convenience pain intensity can be categorized as mild (score 1–1), moderate (score 4–4) and severe for scores 7–7 (Figure 5).
However the ‘Faces Pain Scale’ can be used in child patients for assessing the intensity of pain (Figure 6).
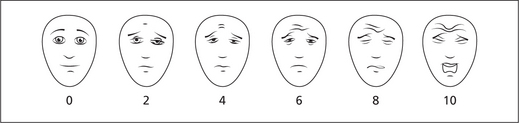
Figure 6 Faces pain scale. Score the chosen face 0, 2, 4, 6, 8 or 10, counting left to right, so ‘0’ = ‘no pain’ and ‘10’ = ‘very much pain’. Do not use words like ‘happy’ and ‘sad’. This scale is intended to measure how children feel inside, not how their face looks. From PAIN, 2001, 93, 173–173 ‘The Faces Pain Scale-Revised: toward a Common Metric in Pediatric Pain Measurement’, by CL Hicks, CL von Baeyer, PA Spafford, I van Korlaar and B Goodenough. Reprinted with permission of the International Association for the study of Pain®.
Intraoral examination should include the evaluation of teeth, periodo/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses