D
dabigatran
Do not confuse Pradaxa with Plavix.
Drug Class:
Anticoagulant, thrombin inhibitor
Indications and Dosages
Precautions and Contraindications
Hypersensitivity to dabigatran or any component of the formulation. May cause fatal bleeding. No specific antidote exists for dabigatran reversal; protamine and vitamin K do not reverse or impact anticoagulant effects of dabigatran. Use in patients with severe renal and hepatic impairment and valvular heart disease is not recommended. Use with extreme caution in elderly patients. Avoid use in patients taking other anticoagulants and P-glycoprotein inducers/inhibitors.
Drug Interactions of Concern to Dentistry
• Increased risk of bleeding: NSAIDs, aspirin
• P-gp inducers (e.g., carbamazepine, barbiturates, St. John’s wort): reduced blood levels and effectiveness of dabigatran
• P-gp inhibitors and CYP3A4 inhibitors (e.g., macrolide antibiotics, azole antifungals): increased blood levels and adverse effects of dabigatran
Drug Class:
Immunosuppressive, IgG1 monoclonal antibody
Dental Considerations
General:
• This is a hospital-type drug, but because some dosing is continued, patients may appear in the dental office while receiving this drug.
• Transplant patients may also be taking cyclosporine and glucocorticoids; review each transplant patient’s medications.
• Short appointments and a reduction protocol may be required for anxious patients.
Drug Class:
Mechanism of Action
A potassium channel blocker that increases conduction of action potentials in demyelinated axons.
Side Effects/Adverse Reactions
Precautions and Contraindications
Hypersensitivity to dalfampridine or its components
Drug Class:
Indications and Dosages
Dental Considerations
General:
Drug Class:
Indications and Dosages
Note: Give initial dose as soon as possible after surgery but not more than 24 hr after surgery.
Serious Reactions
! Accidental overdosage may lead to bleeding complications ranging from minor ecchymosis to major hemorrhage. An unexplained fall in HCT or fall in B/P should lead to consideration of a hemorrhagic event. The antidote protamine sulfate only partially neutralizes danaparoid activity and is incapable of reducing severe nonsurgical bleeding during treatment. If serious bleeding occurs, discontinue danaparoid; give blood or blood product transfusions.
Drug Class:
Drug Class:
Skeletal muscle relaxant, direct-acting
Indications and Dosages
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


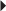 Nonvalvular Atrial Fibrillation
Nonvalvular Atrial Fibrillation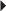 Secondary Prevention of Cardioembolic Stroke or TIA
Secondary Prevention of Cardioembolic Stroke or TIA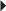 Prevention of Acute Renal Transplant Rejection (in combination with an immunosuppressive)
Prevention of Acute Renal Transplant Rejection (in combination with an immunosuppressive)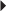 Multiple Sclerosis
Multiple Sclerosis
 Low- to Moderate-Risk Abdominal Surgery
Low- to Moderate-Risk Abdominal Surgery High-Risk Abdominal Surgery
High-Risk Abdominal Surgery Total Hip Surgery
Total Hip Surgery Unstable Angina, Non–Q-Wave MI
Unstable Angina, Non–Q-Wave MI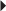 Prevention of DVT or PE in the Acutely Ill Patient
Prevention of DVT or PE in the Acutely Ill Patient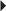 Prevention of DVT
Prevention of DVT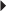 Endometriosis
Endometriosis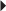 Fibrocystic Breast Disease
Fibrocystic Breast Disease Hereditary Angioedema
Hereditary Angioedema
 Spasticity
Spasticity Prevention of Malignant Hyperthermic Crisis
Prevention of Malignant Hyperthermic Crisis Management of Malignant Hyperthermic Crisis
Management of Malignant Hyperthermic Crisis