CHAPTER 4
Alveolar Ridge Preservation After Tooth Extraction
Maintaining bone quality and quantity in the alveolar ridge during and after tooth removal is critical for assuring good esthetic and functional results and minimizing the need for grafting procedures prior to implant placement.1, 2, 3 Furthermore, preserving the existing bone helps support both fixed and removable prostheses and ensure successful osseointegration of dental implants. Soft tissue contours follow hard tissue contours, so the clinician must not only prevent bone defects but also repair any defects early in the treatment phase. When grafts are necessary, proper technique is critical to the success of the grafts and the final ridge contours.
Alveolar Ridge Resorption
Routine tooth extractions normally result in extraction sockets that heal without any significant difficulties. However, a defect can result from bone naturally growing into the socket and resorbing in height and width during the process.4 A number of different types of bone resorption related to tooth extraction have been identified in the mandible5; however, resorption is most often a concern in the esthetic zone or in areas where the quantity of alveolar bone was minimal before the extraction. The anterior maxillary area is at particular risk because the bone plates are thin and subject to irreversible trauma during tooth removal. 6, 7 In the posterior region, the alveolus has thicker walls and is less prone to resorption.
The crucial issue is how quickly the extraction socket heals and how much connective tissue invades the socket during the process. The eventual shrinkage of the alveolar ridge seems to be related not only to the removal of the tooth, but also to the environment within which the natural healing process of the alveolar defect must occur. Clot retraction and connective tissue regeneration preclude the formation of adequate bone, causing a collapse of the socket and resulting in lost bone contour and poor esthetics.
Atraumatic Tooth Removal
Avoiding tissue loss during tooth removal can help preserve alveolar bone. This goal should be given the highest priority because it may eliminate the need for bone regeneration or grafting later. Additionally, the preservation of hard tissues both complements and bears directly on therapies associated with the treatment of soft tissue defects.8 Some situations can make tooth removal extremely difficult, including the brittle endodontically treated tooth, the severely dilacerated root, and the fractured tooth with little coronal portion to elevate. Most of these indications do not allow for removal and immediate implant placement. Proper instrumentation and technique, however, will allow for the best possible result.
Essential criteria should be followed during tooth removal. A small, sharp peri-otome should be used to incise the periodontal ligament attaching the tissue and tooth. When possible, the interdental papilla should not be reflected, particularly in the esthetic zone. During actual tooth removal, the clinician should use elevators and forceps properly to reduce bony involvement and thus preserve the bone contour. Luxation and controlled force become important allies; however, sectioning the tooth also helps to prevent bone loss. Once the tooth is removed from the socket, soft tissue fragments or pathosis must be removed. The formation of a good blood clot will induce the first stages of bone healing and proper bone fill.
Ridge Preservation
The residual socket can heal uneventfully if the surrounding bone is appropriately thick, tooth removal has been atraumatic, and thick interseptal residual bone is present. In fact, the literature recognizes the legitimacy of implant placement immediately after tooth extraction, given acceptable conditions.9 Many sockets can heal without suturing, but suturing should be accomplished when necessary to avoid damaging or adversely altering the papilla contours. When the socket is compromised, whether as a result of extraction or trauma,10 its walls may be supported with osteoconductive or osteoinductive grafting material.11, 12, 13 Furthermore, a good blood supply is necessary, and obtaining it may require trephining the socket lightly with a small bur to induce bleeding, which can provide a source of osteoprogenitor cells.
When bone grafting is chosen as a restorative method for ridge augmentation, the clinician must decide whether to place the graft and implant together at the time of extraction or to place the implant after the graft has had time to mature. While Becker et al suggest that ridge resorption may not be prevented when implants are placed along with barrier membranes immediately after extraction,9 other research suggests that resorption may be prevented. 14, 15 Individual patient conditions will determine when to place the implant and whether barrier membranes should accompany the graft.
Bone-Grafting Materials
As discussed in detail in chapter 2 and extensively in the literature,16 bone-grafting materials can be divided into three groups: autogenous, allogeneic, and alloplastic or xenograft material. Bone-grafting materials can further be categorized based on four characteristics, all of which are found in the ideal bone graft: osteointegration (ability to bond chemically to bone surface with no layer of fibrous tissue in between), osteoconduction (ability to support surface bone growth), osteoinduction (ability to encourage pluripotential stem cell differentiation), and osteogenesis (ability to form new bone from osteoblastic cells ).17 Autogenous bone material, used with or without the application of complementary therapies such as platelet-rich plasma (PRP),18, 19, 20, 21, 22 is the best and most reliable source for predictable results. Often referred to as the gold standard for grafting, autogenous bone is unmatched in the amount of osteogenic cell viability it offers for bone regrowth and avoidance of histocompatibility problems. For the ridge preservation technique, autogenous bone is often harvested from a secondary intra-oral site, such as the mandibular symphysis or ramus or the maxillary tuberosity.23, 24, 25, 26, 27 Although not a practical approach for small recipient sites, extraoral harvest sites include the iliac crest, rib, cranium, and tibial plateau.28, 29
Allografts, such as demineralized or mineralized freeze-dried bone (DFDBA and FDBA), are often used in place of the autograft because they eliminate the need for a donor site and a second incision and work well when there is a large ratio of host bone to graft material. Allografts are available in powder or putty-like forms; the latter are especially convenient for this application. These materials may induce healing through osteoconduction, osteoinduction, or possibly a combination of both processes.
The third category of graft materials includes alloplasts, which are synthetic bone substitute materials, and xenografts, which are harvested from other species. Alloplastic materials include bioactive glass, glass ionomers, aluminum oxide, calcium sulfate, calcium phosphates, beta tricalcium phosphate, synthetic hydroxyapatite, coralline hydroxyapatite, and calcium phosphate cements.17, 30 Synthetic bone has been shown to prevent ridge resorption after immediate grafting in extraction sites.31, 32, 33, 34 Currently, the most popular xenograft material is bovine in origin. These materials are deproteinated to eliminate their organic properties and to reduce any antigenic potential. Xenografts heal through a process of osteoconduction, acting as a scaffold upon which the patient’s own bone can grow through cellular differentiation from the host blood cells. Xenografts and alloplasts have no inductive potential, meaning they do not induce bone growth on their own. However, a newer variety has been introduced that consists of bovine-derived bone mineral combined with a synthetic short-chain peptide, PepGen P-15 Flow (Dentsply Friadent CeraMed, Lakewood, CO),35, 36, 37, 38, 39 which mimics the cell-binding domain of type I collagen, and is responsible in natural bone for cell migration, differentiation, and proliferation. This material (a hydrogel for easy handling in ridge preservation) may provide the benefits of a synthetic graft containing both an inorganic and an organic component, thus mimicking an autogenous graft material.
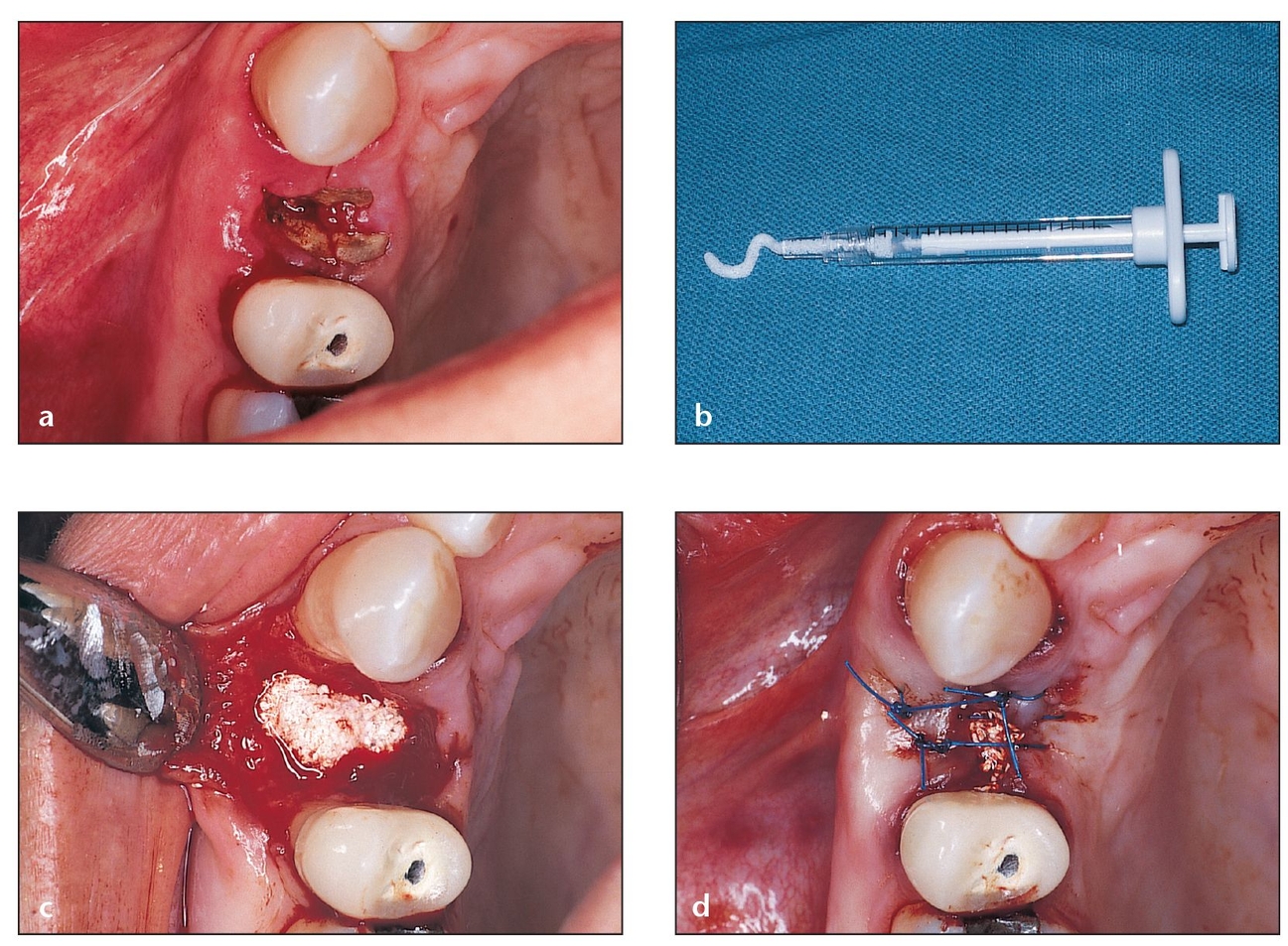
Fig 4-1 Tissue-engineered xenograft (bovine) material in a hydrogel carrier (eg, PepGen P-15 Flow) is a popular grafting material for five-wall defects.
(a) Occlusal aspect of a residual root that should not be removed by conventional procedures such as osteotomy or blunt elevator force, which can fracture the socket walls. Atraumatic extraction is preferred in such cases.
(b) Extraction is performed atrau-matically, leaving the bony walls surrounding the socket intact. PepGen P-15 Flow (particles suspended in a hydrogel) is chosen to graft this socket because there is adequate bony support for the material.
(c) The socket is completely filled to the bone level.
(d) Sutures are used to approximate the flaps as closely as possible. Due to the gel consistency of the material, primary closure is not necessary.
Choosing a Graft Material
One of the difficult aspects of ridge preservation is choosing an appropriate graft material for the specific site. To determine which material is needed, the clinician should evaluate the size and configuration of the defect and then calculate the amount of bone needed to replace the missing tissue. Larger defects require autogenous bone because it provides the maximum amount of cellularity and structure and contains proteins and cells that can cause osteoinduction of new tissue. On the other hand, smaller defects may be appropriate candidates for allografts, alloplasts, or xenografts.
The best time to preserve a ridge or to augment a tooth socket is at the time of tooth removal. Often what seems to be a simple procedure can lead to complicated problems with bone missing from the labial aspect and a compromised esthetic contour. If the lack of bone contour can be determined at the time of initial examination, then scheduling the patient for surgical removal of the tooth and grafting in a single appointment is appropriate. However, tooth extraction and grafting should not be attempted in the presence of suppuration, neoplasms, cysts, or conditions that could cause infection or graft failure.
When evaluating a patient, the clinician must identify the type of defect present; this determination strongly influences the choice of procedure. For example, the clinician can categorize the defect based on the number of walls remaining in the socket, since each of these defect types requires a unique approach to ridge preservation and treatment.
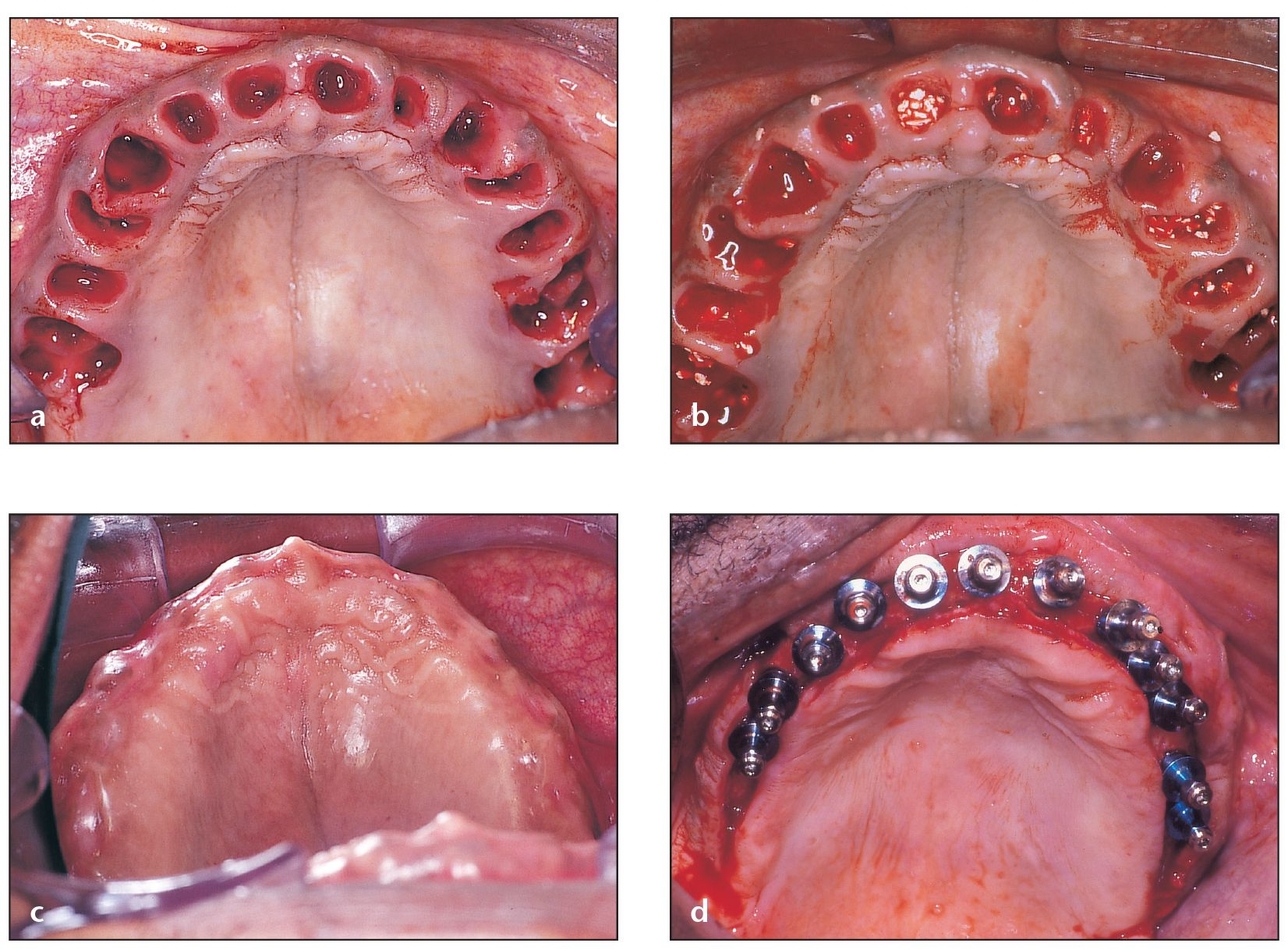
Fig 4-2 Bone grafting at the time of extraction is advised for defects with extremely thin walls.
(a) When planning for future implant placement, multiple extractions show the importance of proper grafting in the healing of sockets.
(b) Filling sockets with PepGen P-15 Flow helps maintain the width and height of the alveolar ridge.
(c) Although some swelling is present 10 days postoperatively, the areas where the implants will be placed appear to be ideal. Ideal ridge width and height for implant placement could not have been maintained without a procedure as simple as socket grafting.
(d) Socket grafting enables 12 implants to be placed in their correct three-dimensional positions that will later carry fixed restorations.
Five-Wall Defects
Extraction sites with five thick bony walls intact may not require any grafting if there is a large amount of interseptal bone present. However, if grafting is indicated or desired at the time of tooth removal, any of the grafting materials in putty or gel consistency may be used for wall support. One of the most popular methods is to use tissue-engineered xenograft (bovine) material in a hydrogel carrier (eg, PepGen P-15 Flow).38 This treatment allows for osteoconductive healing and preserves the tissue contour. The technique requires the clinician to carefully inject the graft material from its dispenser to fill the socket only to the level of bone and then to achieve good closure of the socket’s cervical portion, either by advancement of the soft tissues and closure or by using a collagen plug or barrier. There is typically a 2-to 6-month waiting period before implant placement.
A recent study indicates that enhanced bone formation and faster particle resorption can occur with PepGen P-15 Flow, which consists of particles suspended in a biocompatible inert hydrogel of sodium carboxymethylcellulose, glycerol, and water, compared to the PepGen P-15 particulate (Figs 4-1 to 4-3).40
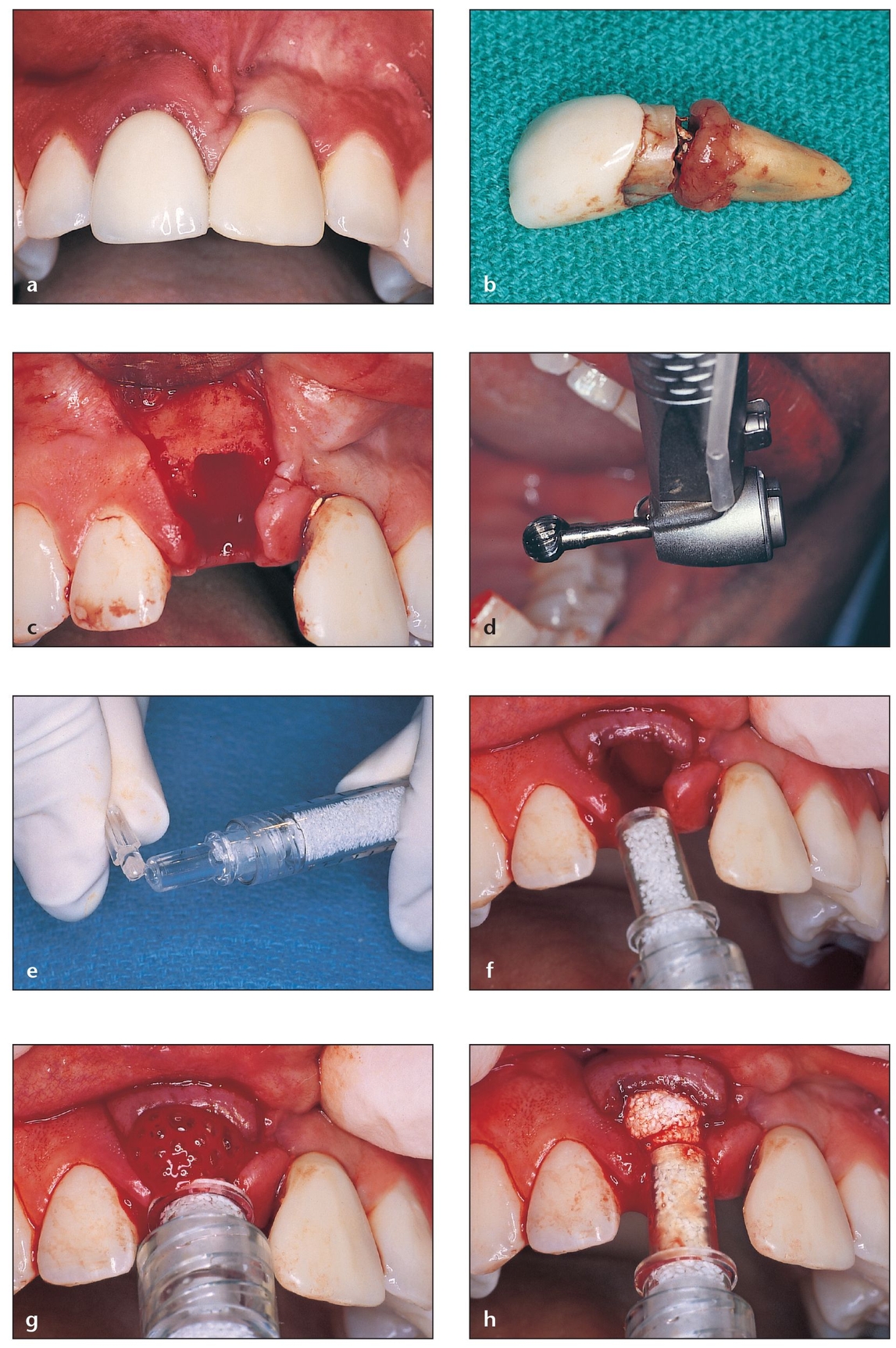
Fig 4-3 PepGen P-15 Flow is used for socket grafting prior to implant placement. This material is reported to enhance bone formation and cause faster particle resorption.
(a) Maxillary right central incisor that requires extraction due to fracture of the root. The maxillary left central incisor is a prosthetic crown over an implant placed 4 years previously.
(b) The fracture is at the cervical third of the root.
(c) After extraction, the socket walls are carefully evaluated to choose the appropriate graft material.
(d) A large, round carbide bur is used inside the socket to eliminate periodontal ligament residue attached to the internal walls.
(e) The cover on the tip of the Flow syringe is removed.
(f) The tip of the syringe easily fits into the defect, allowing the socket to be filled from a more apical position before moving coronally.
(g) The material flows into the socket, facilitating the procedure.
(h) The syringe tip is slowly withdrawn while the Flow is injected to the top of the socket bony walls.
(i) The sutures approximate the flaps that were previously released to avoid dehiscence caused by tension.
(j) Implant placement is successful as a result of previous socket grafting. The buccal wall of the right central incisor will match that of the left central incisor.
(k) Final restoration in place on the implant in the grafted site. Note the maintenance of buccal-palatal width of the ridge.
Four-Wall Defects
For a site that is missing one or two socket walls or that has one or more extremely thin walls, bone grafting is generally advised at the time of extraction. If not grafted, the bone typically narrows as the wound heals, leaving inadequate width for implant placement. Autogenous bone is a good choice for four-wall defects because entire bony walls often must be regenerated. If a sufficient amount of autogenous bone is not available, however, allogeneic bone putty (OrthoBlast II [The Clinician’s Preference, Golden, CO], DBX [Musculoskeletal Transplant Foundation, Edison, NJ], Dyna-Graft II [IsoTis OrthoBiologics, Irvine, CA], or Grafton [Osteotech, Eatontown, NJ]), or a combination of allogeneic bone putty and autogenous bone, can be used (Figs 4-4 to 4-8).41, 42, 43, 44, 45, 46, 47, 48 The clinician must use a graft material with sufficient body to reconstruct the missing or thin wall (typically the facial wall) and to support the overlying soft tissues. Some clinicians use a barrier for guided bone regeneration (GBR) in an attempt to enhance the graft and prevent soft tissue ingrowth, but simply advancing the flap to obtain primary closure is generally sufficient.49
OrthoBlast II is a synergistic combination of demineralized bone and cancellous bone in a reverse-phase medium. OrthoBlast II is made of demineralized human bone that has passed a validated in vitro assay for osteoinduction. Because it is made of cancellous bone, it can provide an osteoconductive scaffold for bone deposition and remodeling. Moreover, its unique reverse-phase medium carrier becomes more viscous at body temperature, providing exceptional handling and ease of use with minimal loss through irrigation and suction. OrthoBlast II is available in 1-and 3-mL prefilled syringes and in a 5-mL container for larger grafting procedures.
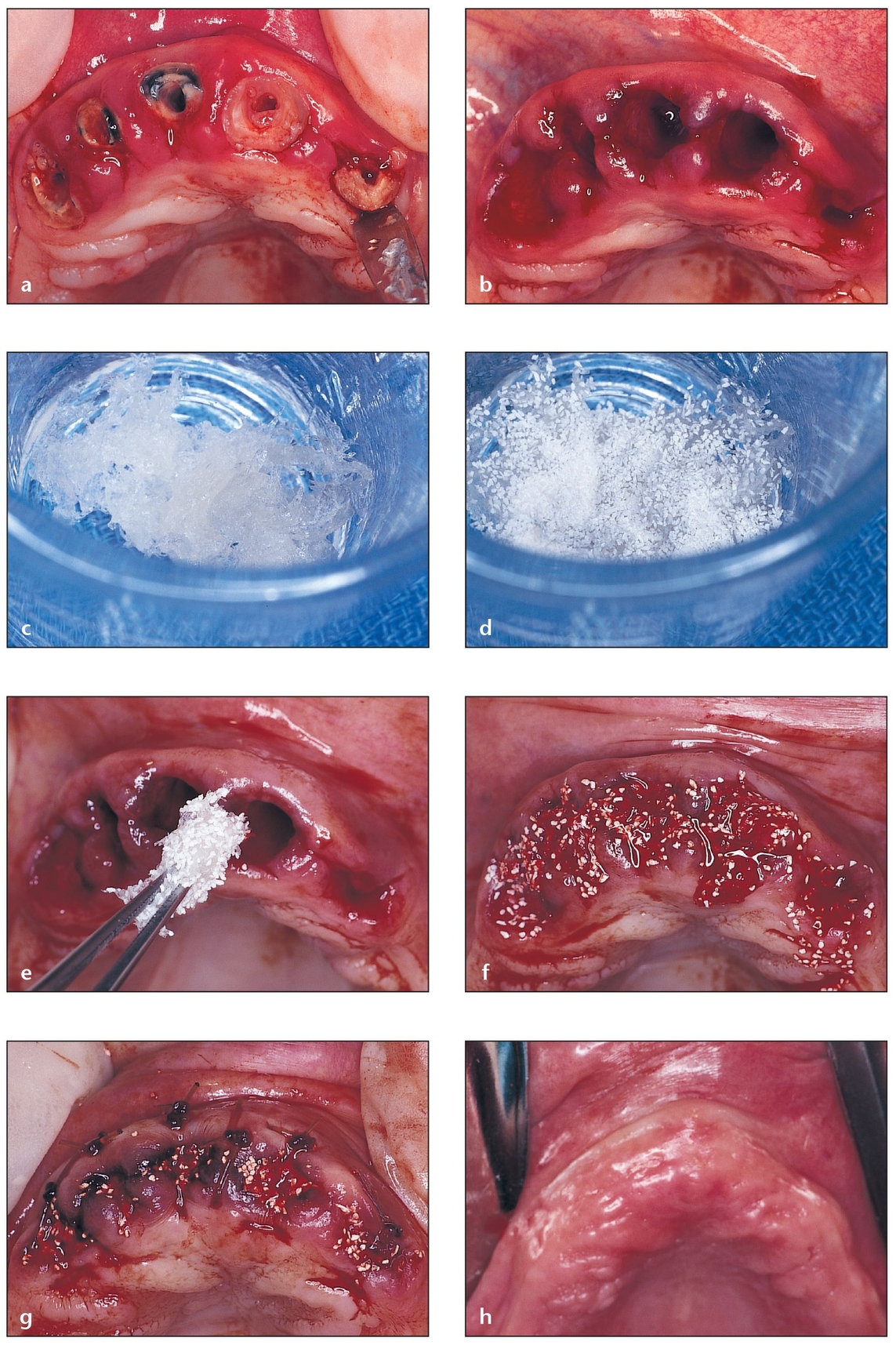
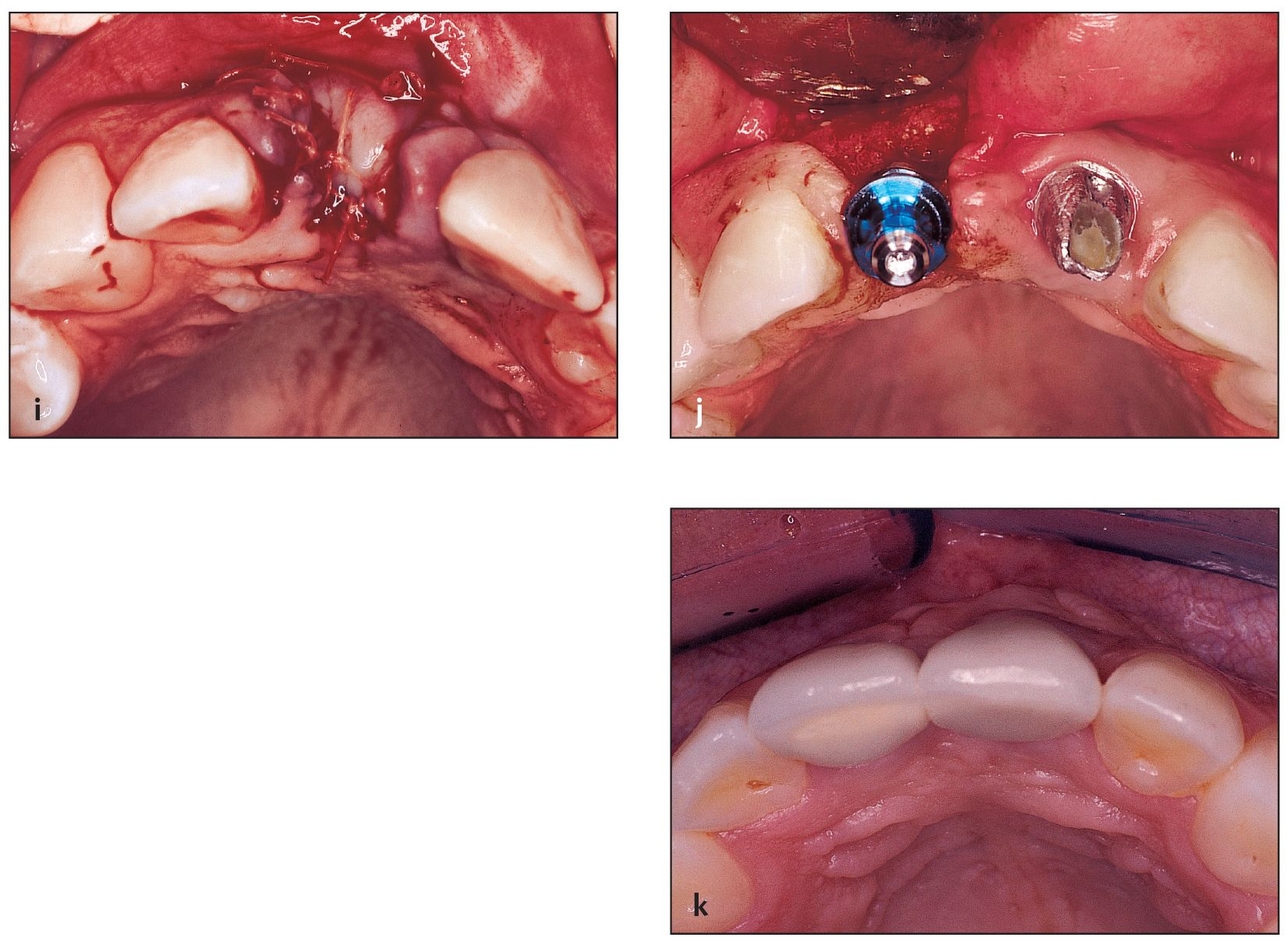
Fig 4-4 Allogeneic bone putty is used for socket grafting of a four-wall defect.
(a) A failing fixed partial denture is removed, revealing unrestorable roots. A closer look reveals that atraumatic root extractions and socket grafting are needed.
(b) Thin buccal wa/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


