Site Selection, Evaluation and Imaging for Dental Implants
Dental implants are prosthetic devices implanted into the oral tissues either beneath the mucoperiosteal layer or in the bone to provide retention and support for the fixed or removable prosthesis. Although fabrication of dental implants dates back to many decades, the advent of dental root form implants (DRFIs) have paved way for universal acceptance of implants as replacement for lost teeth. The dental implant restorations not only enhance the esthetics for the patients, but also improve the oral function for speech and mastication. Hence, the dental root form implants (Figure 1) gained immense popularity among the dental practitioners as well as the patients in the dental profession. Nothing is more important in the dental implantology than the precise evaluation and placement of the implant structures in the maxilla and mandible. In order to do this effectively, one needs to precisely understand the surgical anatomy of the maxillofacial region and the anatomical variations that might be encountered. Secondly, they need to grasp the biomechanics of the dental implant material. Fortunately, now, the science behind the dental implantology is strong, backed with research and evidencebased trials. The fruits of the decades of clinical trials using various dental implant materials along with numerous loading protocols have refined the predictability of dental implants for the maxillofacial region.
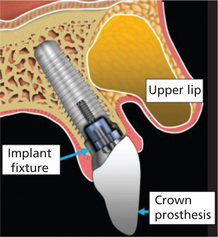
Figure 1 Diagrammatic representation of the typical implant fixture and the crown properly placed within the maxillary alveolus
Historical Perspectives
Historically, there were three distinct forms of dental implants, namely, the subperiosteal (epiosteal), the transosseous (staple bone or transmandibular) and the endosteal (blade or plate, ramus-frame and root form) types. Dental practitioners should be familiar with the radiographic appearances of older dental implant systems although many of them are rarely used after the advent of the dental root form implants.
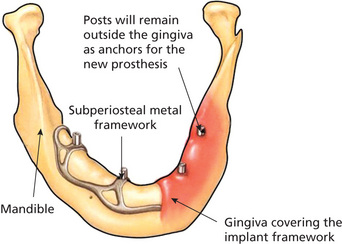
Subperiosteal implants
Subperiosteal implants are metallic meshes that are custom-built to fit over the alveolar processes and are located underneath the periosteum (Mupparapu and Beideman, 2000). Direct bone impressions via surgery would lead to the fabrication of mesh in a laboratory that fits under the mucoperiosteum (Figure 2). The framework normally rests on the mandible with no penetration into the bone. Two surgeries were involved in the fabrication of these implants. First surgery is for the impression and the second surgery is for the placement of the mesh. The first subperiosteal dental implant in the world was placed by George Dahl in Sweden. The first American subperiosteal implants were developed in 1947. Gershkoff and Goldberg placed the first subperiosteal complete denture implant manufactured with Vitallium (Moore and Hansen, 2004). Dr Leonard Linkow of New York made significant changes to the original design by incorporating fenestrations into the buccal peripheral struts which made it possible for the mucoperiosteum to reattach the bone in between these fenestrations. He also holds 35 patents on various designs of subperiosteal and oral implants. Multiple metallic posts extend from the mesh into the oral cavity crossing the mucoperiosteal barrier to support the prosthesis. Mandibular subperiosteal implants have been shown to be successful in many clinical studies and some of the implants reviewed were present in the mouth for more than 10 years. The success rate for these implants varied in many studies with higher success rate for 10-year periods and lower success rates for longer periods (Moore and Hansen, 2004). Computed tomography (CT) scans were also used to allow CAD/CAM fabrication of the framework, eliminating the need for impressions via surgery. Subperiosteal implants are no longer preferred after the success of endosteal implants although one can occasionally see them in clinical practice with varying degrees of presentation (Beddis et al, 2012).
Endosteal implants
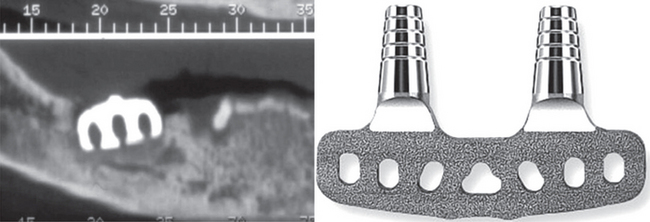
There are three forms of endosteal implants: the root form, the ramus frame and the plate or blade form implants. Blade implants are rectangular in shape similar to a razor blade. These are used over horizontal column of bone. One or more posts usually extend into the oral cavity to permit fixation of the prosthesis. The only blade implants that are seen clinically at present are the previously placed implants that either failed or fractured and the patient reported for treatment. One such example where the blade implant failure led to the osteomyelitis in the mandible is shown in Figure 3. The root form implants can be either cylinder-type, screw root type or a combination of both. These implants use the vertical column of bone unlike the blade implants.
Indications for Dental Implants
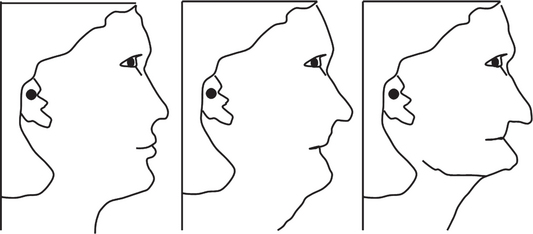
Patients who lost the entire dentition will show significant changes to the facial profile after loss of teeth chiefly due to the alveolar resorption and the loss of vertical dimension (Figure 4). Restoration of function and esthetics would then become the goal for any prosthesis without compromising on the masticatory efficiency.
Patient Management with Dental Implants
There are three aspects of patient management that the practitioner should remember when planning treatment for dental implants:
Identification of the Implant Site
Selection of the patient
Patient selection is a key factor in going forward with the identification of future implant site(s). This essentially boils down to the assessment of the medical and dental status of the patient. If the patient’s medical and dental history precludes a dental implant placement and a good prognosis, then that should be communicated to the patient ahead of time. Systemic health of the patient plays a vital role in the overall selection of the patient. Any medical condition that delays or alters wound healing should be identified first. A good example for this is ‘diabetes mellitus’. Uncontrolled type 1/type 2 diabetes or untreated diabetes usually results in delayed wound healing. Diabetes (type 1 and type 2 combined) affects approximately 25.8 million people in the Unites States alone. There are at least 79 million people who are considered prediabetics (National Diabetes Fact Sheet, 2011, Centers for Disease Control and Prevention [http://www.cdc.gov/diabetes]). Worldwide, as per 2011 World Health Organization estimates, around 346 million people have diabetes (http://www.who.int/mediacentre/factsheets/fs312/en/index.html).
Absolute contraindications
5 Active radiation and/or chemotherapy
Both ionizing radiation and chemotherapy disrupt hematopoiesis, and implantation should be deferred in such situations as wound healing is delayed. In addition, if the salivary glands are involved in the line of fire, there would be substantial xerostomia which in turn, would contribute to poor oral hygiene and increase in dental caries secondary to xerostomia. Studies have shown that in about 3–35% of patients, spontaneous or traumatic osteoradionecrosis would be a complication (Marx and Johnson, 1987). When the patient is on cytotoxic anticancer drugs, granulocytopenia followed by thrombocytopenia are expected which might lead to infection, hemorrhage, mucositis and pain. Implant therapy should be deferred until after the patient is completely off the cytotoxic medication.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses