Chapter 3
Periodontal Risk Factors and Modification
INTRODUCTION
As a common chronic disease of the oral cavity, periodontal disease is a set of inflammatory conditions affecting the supporting structures of the dentition (Armitage, 1999). After its initiation, the disease progresses with the loss of collagen attachment to the root surface, the apical migration of the pocket epithelium, the formation of deepened periodontal pockets, and the resorption of alveolar bone. Untreated periodontal disease continues with progressive alveolar bone destruction, leading to increased tooth mobility and potential tooth loss (Page and Kornman, 1997).
Reports from epidemiological studies, analysis of tissue histology, clinical trials, and animal experiments consistently demonstrate a multi-factorial aetiology of periodontal disease. Chronic periodontitis is the most prevalent form of destructive periodontal disease (Albandar et al., 1999). Furthermore, cross-sectional and longitudinal data from epidemiological research in periodontology suggest that risk factors can be identified, and that some of these factors could be controlled to prevent the development and progression of the disease. Risk factors are part of the causal chain of a particular disease or can lead to the exposure of the host to a disease (Consensus Report, Annals of Periodontology, 1996). The presence of a risk factor implies a direct increase in the probability of a disease occurring, and if absent or modified, a reduction in that probability should occur. Risk factors are generally classified as modifiable and non-modifiable. While gender, age, and ethnicity are non-modifiable, insufficient oral hygiene or tobacco use are identified as modifiable risk factors for periodontal disease.
A risk factor may be modified by interventions, thereby reducing the probability that a particular disease will occur. However, the susceptibility to a specific disease may vary among different individuals exposed to a given risk factor over time. Additionally, cumulative interactions between both modifiable and non-modifiable risk factors, described as “complex risk factors,” have been suggested (Stolk et al., 2008).
A variety of interrelated risk factors may influence both the onset of periodontal disease and its progression (Figure 3.1). The detection of periodontal disease progression remains challenging since it typically relies on the comparison of measurements made with a calibrated periodontal probe and non-standardized periapical radiographs over time. Some emphasis should be placed on the early identification of periodontal risk factors to assess the likelihood of periodontal disease progression in susceptible individuals since both methods detect periodontal breakdown only after it has occurred. The goal of this chapter is to discuss known non-modifiable and modifiable risk factors as well as their management in the dental practice to provide prevention and a careful maintenance program for the periodontal patient while following the best available evidence today.
NON-MODIFIABLE RISK FACTORS
Genetic and Hereditary Factors
Periodontal diseases are shown to be affected by genetic factors (Page and Kornman, 1997). A number of genetic disorders, such as Down syndrome, leukocyte adhesion deficiency syndrome (LADS), Papillon Lefevre syndrome, Chediak Higashi syndrome, chronic neutrophil defects, or cyclic neutropenia are associated with more or less severe periodontal conditions.
The hereditary aggregation was demonstrated in a twin study for chronic periodontitis (Michalowicz et al., 1991) and an epidemiological trial on aggressive periodontitis in a Dutch population (van der Velden et al., 1993). Following the adjustment for environmental factors such as tobacco use, it was estimated that 50% of the variance in disease may be attributed to a genetic background (Michalowicz et al., 2000).
Practical Application of Genetic Susceptibility Testing
During periodontal inflammation, inflammatory cytokines, including interleukin-1 β (IL−1β) and tumor necrosis factor-α (TNF-α), activate catabolic enzymes such as matrix metal-loproteinases, subsequently leading to the breakdown of connective tissue. Any gene polymorphism of such proteins potentially alters the susceptibility of the host to periodontal diseases. A single nucleotide polymorphism (SNP) is a mutation that occurs when a single nucleotide is altered within its genome due to changes in the base pair sequence.
Past periodontal diagnostic research has focused on evaluating several selected candidate gene SNPs including variations of IL-1β or TNF-α. The evidence-based perspective: Evidence from a review suggests that some polymorphisms in the genes encoding interleukins (IL)-1, Fc gamma receptors (FcgR), IL-10, and the vitamin D receptor may be associated with periodontitis in certain ethnic groups (Loos et al., 2005). In addition, the association of the composite IL-1 genotype with periodontitis progression and/or treatment outcomes was analyzed using a systematic review approach (Huynh-Ba et al., 2007). There is limited evidence for such an association. Thus far, no specific genetic risk factor for periodontitis has been identified (Loos et al., 2005). Therefore, the clinical relevance of different commercially available genetic tests is limited.
Figure 3.1. Interplay of modifiable and non-modifiable risk factors with the pathogenesis of periodontal diseases.
Gender
Hormonal changes in women during menstruation, pregnancy, menopause, or therapy with pharmaceutical supplements have an impact on periodontal health. Disease susceptibility may be increased due to hormone-related alterations of the gingival blood flow (Kovar et al., 1985), the composition and flow rate of saliva (Laine, 2002), or the bone metabolism (Lerner, 2006). Additionally, data from epidemio-logical studies interestingly reveal that men may be at greater risk for periodontal diseases: in most clinical trials men are often found with worse periodontal health (Albandar, 2002; Meisel et al., 2007). Most often, however, these deteriorations may be explained by an increased prevalence of male tobacco use and mens’ increased tendency to neglect oral hygiene (Meisel et al., 2007).
Gender-specific Practical Applications
Periodontal diseases have been associated with gender-specific complications such as an increased susceptibility for gingivitis during pregnancy (Russell and Mayberry, 2008), pre-term delivery, or low birth weight (Madianos et al., 2002). Therefore, gender-specific periodontal disease risk factors should be assessed in women by all oral health professionals (Krejci and Bissada, 2002). In pregnant women, a rigid recall interval including oral hygiene motivation is recommended. Consequently, it is suggested that existing periodontal inflammation should be treated before pregnancy.
Factors that are increasingly investigated in recent studies include gender-specific diseases such as osteoporosis or metastatic bone disease in relation with hormone substitute therapy or bisphosphonate medication in post-menopausal women (Diel et al., 2007; Payne et al., 1999). Bisphosphonates affect osteoclast functions, leading to the inhibition of physiological bone remodeling. With both surgical and non-surgical periodontal treatment, a bisphosphonate-associated osteonecrosis of the jaws should be considered as a complication. In one prospective cohort study of osteoporotic women in early menopause, it was found that a supplementation of estrogen may be associated with reduced gingival inflammation and impaired clinical attachment loss (Reinhardt et al., 1999).
Age
The aging process itself is suggested to be an independent risk factor for periodontal diseases (Papapanou et al., 1989). In contrast, a longitudinal study involving an elderly Scandinavian population (age 75 or older) demonstrated stable periodontal conditions for five years, suggesting a limited impact of the aging process itself in otherwise relatively healthy individuals (Ajwani and Ainamo, 2001). However, the extent and severity of periodontal diseases are shown to increase with age (Albandar, 2002) as a consequence of the cumulative burden from various risk factors such as tobacco use or plaque accumulation (Albandar, 2002; Albandar et al., 1999). Additionally, metabolic disorders, including diabetes mellitus, osteoporosis, rheumatoid arthritis, or vascular diseases, are more likely to develop in the elderly and thus affect periodontal conditions (Persson, 2006).
Age-specific Practical Applications
Life expectancy has increased significantly over the past few years in industrialized countries (Holm-Pedersen et al., 2005). As compared to previous populations, the elderly population is retaining its natural dentition, potentially leading to more periodontal problems. The presence of various chronic diseases, such as diabetes mellitus or specific medications (e.g. Vitamin K antagonists), may also interact with the periodontal condition or the treatment. Thus, there is a need for multi-disciplinary treatment in many cases, due to the increased likelihood of co-morbidity in the elderly (Persson, 2006). Aging is often associated with the individual’s impaired mobility, probably leading to an incapacity for regular supportive periodontal treatment. A close interplay with nursing homes or public oral health care providers may be advisable. Physical or mental disorders may affect the effectiveness of supragingival plaque control. The suggestion of simple interventions, including the weekly use of chlorhexidine-containing mouth rinses in conjunction with cognitive behavioral interventions, might be useful (Hujoel et al., 1997).
MODIFIABLE RISK FACTORS
Insufficient Oral Hygiene
Today, accumulated plaque is considered to be a dental biofilm briefly defined as a complex bacterial structure adherent to wet surfaces (Socransky and Haffajee, 2002). For the therapy of periodontal diseases, it is important to consider that biofilms can protect their microorganisms, either from the host’s immune response or antimicrobial agents, and thus become difficult therapeutic targets (Socransky and Haffajee, 2002). So far, only mechanical debridement was shown to be a predictable approach to successfully destruct the dental biofilm. Therefore, mechanical plaque control should be performed supragingivally by the individual on a regular basis and subgingivally, if needed, by the oral health professional.
Any factors that facilitate biofilm formation, such as plaque retention or insufficient supragingival plaque control, are common risk factors for periodontal breakdown due to their causality with gingival inflammation and possibly the onset of periodontitis. This includes several anatomic conditions, such as enamel pearls, tongues, grooves, root furcations, and concavities, as well as root proximities (Roussa, 1998; Vermylen et al., 2005). Calculus and acquired iatrogenic factors, such as insufficient restorations, additionally contribute to plaque accumulation (Lang et al., 1983; Oliver et al., 1998).
It was proven some 40 years ago by classical experiments conducted by the work group Lüe and Theilade that oral micro-organisms are relevant for the development of inflammable periodontal diseases (Theilade et al., 1966). In a longitudinal study of more than 26 years, a further research group examined the influence of plaque-induced gingival inflammation on the subsequent loss of clinical attachment in a periodontally well-maintained Scandinavian population (Schatzle et al., 2003). The supragingival plaque accumulation correlated with the degree of gingival inflammation. However, sites with bleeding on probing at every visit demonstrated about 70% more attachment loss than sites without inflammation for the duration of the study. Moreover, it was recently shown that the susceptibility of gingivitis seems to be higher among males suffering from periodontitis (Dietrich et al., 2006).
Practical Application of Microbiological Testing
A multitude of different microbiological tests, based on morphological, enzymatic, cultural, genetic, or antigenetic bacterial properties, are available for both qualitative and quantitative microbiologic risk assessment of periodontitis. In many clinical situations, however, these tests fail to provide evidence-based recommendations for therapy (Sanz et al., 2004). Nevertheless, in a few cases, microbiologic tests can support treatment planning, including cases resistant to combined mechanical-antibiotic therapies, e.g. scaling and root planing, and the prescription of metronidazole and amoxicillin.
Tobacco Use
Earlier publications confirmed tobacco consumption as a risk factor for periodontal diseases. Over the past few years, oral health research has significantly contributed to the understanding of the mechanisms leading to the deterioration of the hard and soft tissues supporting the teeth. With the recording of the number of cigarettes smoked per day, the number of years tobacco was used, and the amount of time since tobacco use cessation, a dose response relationship was established using the Comprehensive Smoking Index (Dietrich and Hoffmann, 2004).
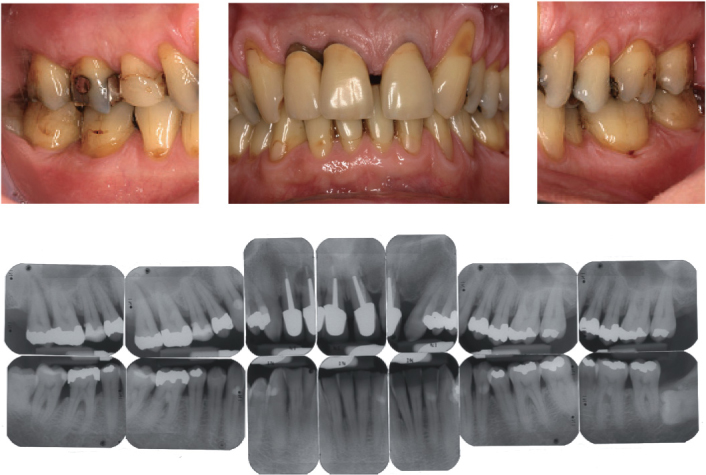
With increased use of tobacco, patients show higher periodontal probing depths, increased clinical attachment loss, more alveolar bone resorption, a higher prevalence of gingival recessions, and a higher risk for tooth loss (Tonetti, 1998). In contrast to this, with smokers, the clinical characteristics of gingival inflammation or bleeding on periodontal probing are less established (Dietrich et al., 2004). Smokers show less positive results after conventional, surgical, and regenerative periodontal therapy. The benefits of mucogingval surgery are reduced and less successful in smokers (Erley et al., 2006). Moreover, smoking impairs the osseointegration of oral implants and is at least partly responsible for a majority of biological complications in implant dentistry, such as peri-implantitis (Strietzel et al., 2007). Based on the present understanding of periodontal diseases, the clinical findings, and the specific therapeutic outcomes with smokers, it appears to be reasonable, next to the current classification of periodontal diseases, to use the term “smokers periodontitis” (Figure 3.2).
Figure 3.2. Clinical and radiographic images of a 45-year-old male smoker. Typical signs of smoker’s periodontitis (20 pack years) including attachment loos, gingival recessions, and radiographic alveolar bone loss, particularly in the maxillary and mandibular front area.
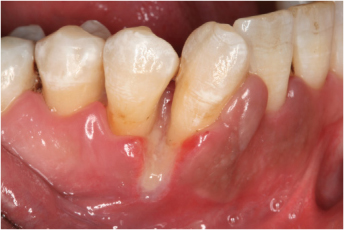
A common clinical observation is delayed wound healing after therapeutic interventions (Silverstein, 1992) (Figure 3.3).
There are various potentially significant pathogenic effects of tobacco-related substances on the periodontal tissues, immune response system, or composition of the oral flora. Periodontal destruction associated with tobacco use is caused by a wide multidimensional range of effects on different functions in cells, tissues, and organ systems. Some of these effects are diametric in nature, due to the effects of different tobacco constituents. However, when summarizing the properties of the tobacco-induced alterations in the metabolism of vasculature, connective-tissue, and bone, as well as on cell-mediated and humoral immunity, it is more than likely that tobacco use shifts the physiological balance between anabolic and catabolic mechanisms in a more destructive direction, due to an alteration of protective immune and tissue mechanisms (Johnson and Guthmiller, 2007; Palmer, 2005; Ryder, 2007). Moreover, there is evidence that tobacco consumption may change the genetically determined susceptibility for periodontal diseases (Meisel et al., 2004).
The evidence-based perspective: There is robust evidence from a systematic review (Bergstrom, 2006) that smoking is a strong risk factor for periodontal diseases. On the basis of 70 cross-sectional studies, 14 case-control studies, and 21 cohort studies, it is concluded that smoking negatively interferes with a healthy periodontal condition.
Diabetes Mellitus
Diabetes mellitus is a metabolic disorder categorized by a hyperglycemia due to impaired insulin production or insulin resistance. Insulin is a pancreatic-hormone-maintaining glucose metabolism. At least two major groups of diabetes mellitus (type 1 and type 2) are differentiated based on their pathogenesis. In addition, some diseases such as hormone-secreting tumors, conditions such as pregnancy (gestational diabetes), or drugs such as corticosteroids can lead to diabetes mellitus. Treatment of diabetes mellitus primarily aims to keep blood sugar levels within a normal range. Treatment may include an interview for behavioral change for dietary adjustment, physical activity, or several drugs. They usually include oral antihyperglycemic drugs or insulin replacement therapy, as well as drugs for prevention and/or treatment of diabetes complications such as hypertension. If left untreated, serious long-term complications may occur, affecting small and large blood vessels, eyes, kidneys, nerves, or the immune system.
Figure 3.3. Impaired wound healing in a female smoker (age 44, 45 pack years) seven days following periodontal non-surgical debridement.
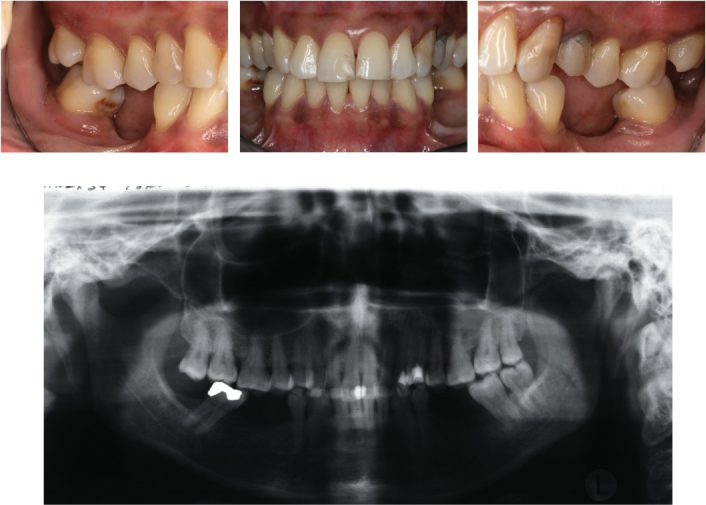
Diabetes mellitus has been associated with increased prevalence and severity of periodontal disease (Figure 3.4) (Emrich et al., 1991; Shlossman et al., 1990). The majority of studies demonstrate a more severe periodontal condition in diabetic adults than in adults without diabetes (Papapanou, 1996; Verma and Bhat, 2004). The type of diabetes does not affect the extent of periodontitis when the duration of diabetes is similar. However, type I diabetics develop the disease at an earlier age, and, hence, have it for longer periods, and may therefore develop a greater extent and severity of periodontitis (Oliver and Tervonen, 1994; Thorstensson and Hugoson, 1993). Well-controlled diabetics are more likely to be similar to non-diabetics in their periodontal status (Westfelt et al., 1996).
A common complication of diabetes mellitus is the increased susceptibility for microbial infections due to an impaired function of the host immune response. Diabetes mellitus may contribute to periodontal inflammation via specific mechanisms. The hyperglycemia may promote the formation of advanced glycation end products (AGE), i.e., glycated body proteins (Wautier and Guillausseau, 1998). Accumulation of AGE may have an impact on periodontal micro-vascularisation or may lead to an increased number of monocytes within the site of inflammation (Katz et al., 2005). A modification of physiologic cell functions of certain subtypes of granulocytes is also reported (Manouchehr-Pour et al., 1981a; Manouchehr-Pour et al., 1981b). Moreover, some studies suggest an alteration of pro-inflammatory mediators in gingival crevicular fluid, including tumor necrosis factor-α, prostaglandin-E2, and interleukin-1β (Engebretson et al., 2004; Salvi et al., 1997a; Salvi et al., 1997b). A further research group reported a decreased gene expression of anti-inflammatory and anti-bone-resorptive molecules such as interleukin-10 and osteoprotegerin (Duarte et al., 2007). Collagen is produced by fibroblasts and is an important molecule of the periodontium. In-vitro findings indicate a reduction of collagen synthesis in a dose-dependent fashion of glucose concentration (Willershausen-Zonnchen et al., 1991).
Interestingly, there is some evidence for periodontitis as a contributing factor in the pathogenesis of diabetes mellitus (Taylor et al., 1998). Inflammatory markers, including tumor necrosis factor-α, increase with periodontal severity and thus affect the insulin metabolism in diabetics (Engebretson et al., 2007). In contrast, their reduction occurs following antimicrobial periodontal therapy, leading to an improvement of glycemic control (Iwamoto et al., 2001).
The evidence based perspective: Evidence from a systematic review, including meta-analysis, suggests a significantly higher severity but the same extent of periodontal disease in diabetics compared with non-diabetics (Khader et al., 2006).
Stress
Stress may be caused by acute or chronic stressors. A stressor can be intrinsic or extrinsic in origin and is frequently defined as anything that causes an adaptive and non-specific neurological and physiological response in an individual. Chronic stressors are of relatively longer duration and include several “life events” such as the loss of a family member, splitting of a relationship, long-term illness, miscarriage, or “daily hassles.” Events of a relative short duration, such as traffic jams, surgical interventions, dental visits, or unpleasant questions in a medical exam, on the other hand, may act as acute stressors to an individual. The physiologic response is mediated by several immune-to-brain-to-immune regulatory pathways (Breivik et al., 2006). The individual stress coping behavior depends on genetic susceptibility and environmental and developmental factors as well as gathered experiences during the course of life.
Several studies indicated an association of negative stress, depression, anxiety, or poor coping behavior with periodontal diseases (Genco et al., 1999; Hugoson et al., 2002; Wimmer et al., 2002). Negative stress may lead to an increased susceptibility to periodontitis mediated through different pathways.
Figure 3.4. Clinical and radiographic images of a 46-year-old female patient with type II diabetes mellitus and chronic periodontitis. The metabolic disease was diagnosed in 1993 and is well controlled (level of blood sugar glucose 6, 3 mmol/l) by the physician. The patient receives oral antidiabetic drugs.
As one mechanism, it was suggested that due to stress, the oral hygiene may be limited (Dein/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


