CHAPTER 3
Barrier Membranes for Guided Bone Regeneration
With the advent of barrier membrane techniques and materials, more predictable restoration of the architecture and function of the bone and periodontium are being achieved through guided tissue regeneration (GTR) and guided bone regeneration (GBR). Barrier membrane techniques are based on criteria that reflect the biologic behavior of different tissues (eg, gingival epithelium, connective tissue, periodontal ligament, alveolar bone) during wound healing.1 The purpose of barrier membrane procedures is selective cell repopulation—to guide proliferation of the different tissues during healing after therapy (Fig 3-1).2 Cells that have the ability to form bone, cementum, and periodontal ligament must occupy the defect to stimulate tissue regeneration. The progenitor cells reside in the periodontal ligament and/or alveolar bone, both of which remain around the tooth or bony defect (Fig 3-2).3 Placement of a physical barrier between the gingival flap and the defect before flap repositioning and suturing prevents the gingival epithelium and connective tissue (undesirable cells) from contacting the space created by the barrier. The barrier also facilitates repopulation of the defect by regenerative cells.4, 5, 6, 7
Although most early studies were concerned with the treatment of periodontal defects, the principal objectives of barrier membrane techniques are to facilitate augmentation of alveolar ridge defects, improve bone healing around dental implants, induce complete bone regeneration, improve bone-grafting results, and treat failing implants.8, 9, 10, 11, 12, 13, 14 Barrier membrane techniques, or osteopromotion procedures, use a barrier to prevent other tissues, especially connective tissue, from entering the intended site of bone reformation and from interfering with osteogenesis and direct bone formation.9 Membranes may also provide additional wound coverage, acting as a duplicate surgical flap to provide added stability and protection of the blood clot and preventing ruptures along the interface between the healing tissues and the root surface.15 In addition, membranes may also provide a tent-like area for the blood clot, creating a space under the surgical flap that will act as the scaffold for the ingrowth of cells and blood vessels from the base of the lesion.16
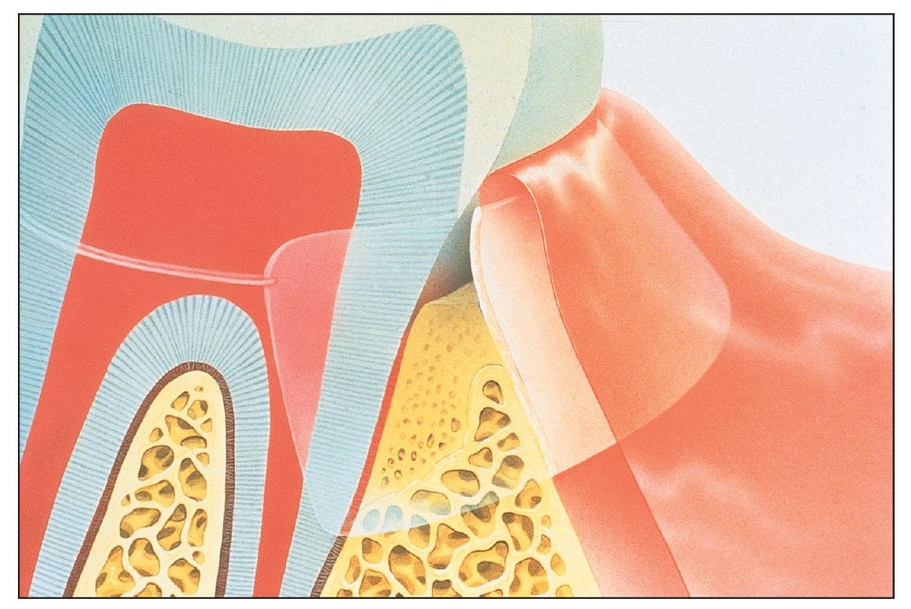
Barrier membranes are placed to prevent undesirable tissues from invading the defect and to protect and promote desirable tissue formation within the defect.
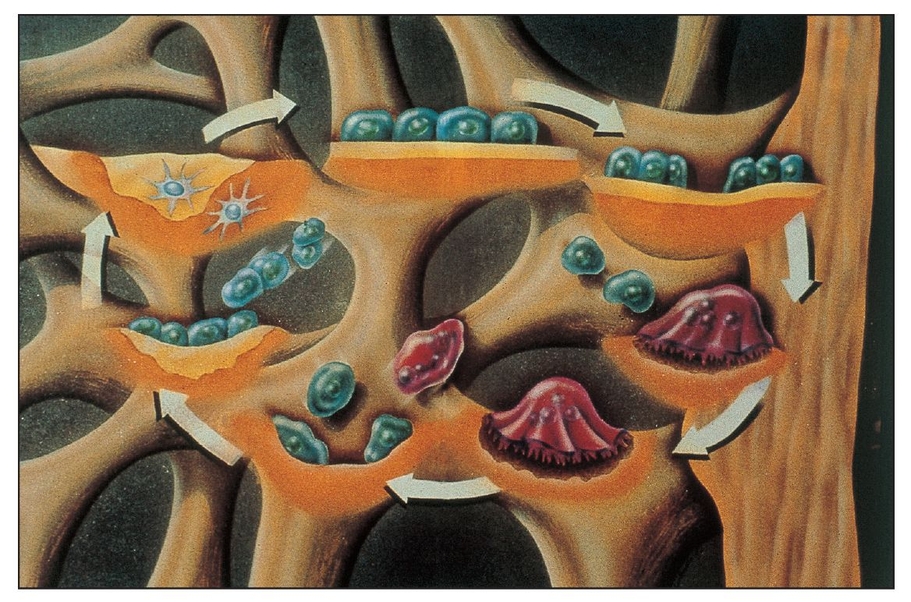
Barrier membranes can protect and isolate progenitor cells in the periodontal ligament and alveolar bone.
Studies have shown that interacting factors influence the predictability of periodontal procedures; tissue separation is just one of these factors.17, 18 The main objective of barrier membrane procedures is to create a suitable environment in which the natural biologic potential for functional regeneration can be maximized.18 Creating and maintaining a blood clot–filled space, preventing inflammation as a result of bacterial invasion, isolating the regenerative space from undesirable tissues, and ensuring the mechanical stability of the resolving wound complex are some of the most important factors for creating a suitable environment for regeneration.
The final goal of barrier membrane technologies is the restitution of the supporting tissues (ie, bone) that were lost as a consequence of inflammatory disease or trauma.19, 20 Several treatment modalities have been used in an attempt to reach this goal, with or without the placement of bone grafts or bone substitutes.19, 21
Materials Used for Barrier Membrane Techniques
Different types of membrane materials have been developed concomitant with the expansion of barrier membrane techniques and their clinical applications.8 The biomaterial and physical characteristics of the membranes that are used can significantly influence barrier function.18 Biocompatibility, cell occlusiveness, space making, tissue integration, and clinical manageability are criteria that must be considered in the design of materials used for regenerative procedures.22 These materials should also be safe, efficient, cost effective, and easy to use. In addition, they must remain in place until regeneration is complete and must not interfere with newly formed tissue.23, 24
The clinical and histologic results of studies using various barriers have generally been favorable. However, no single material has been found to be ideal for every clinical situation because each type has specific benefits and certain associated drawbacks or limitations.18, 25, 26 To ensure success, it is important to know the advantages and disadvantages inherent to each material for the application in which it is being used.18 For example, pin fixation systems can be used to stabilize membranes in some situations, whether or not bone graft procedures are included.27, 28 Generally, membrane barrier materials are divided into two categories, nonresorbable and resorbable. In addition, attempts have been made to use alternative materials, such as titanium foil, to gain the stability of a nonresorbable membrane without the need for membrane removal.29
Nonresorbable Membranes
The earliest studies used nonresorbable materials, such as cellulose filters (Millipore Filter, Millipore, Bedford, MA) and expanded polytetrafluoroethylene (e-PTFE) (Gore-Tex, W. L. Gore, Flagstaff, AZ). These materials were not originally manufactured for use in medical or dental procedures (Fig 3-3). Cellulose filters and e-PTFE were chosen as barrier materials because they allowed the passage of liquid and nutritional products through the barrier, but their microporosity excluded cell passage.2 An in vitro study concluded that the Millipore filters enhanced early osteoblast (MC3T3-E1) attachment.30 Another nonresorbable membrane barrier that has received considerable attention in the literature is a simple dental rubber dam.
Cellulose filters
Initial studies examined the use of cellulose filters in primates to exclude connective tissue and gingival epithelium, allowing cells from the periodontal ligament to repopulate the wound.31 The periodontal ligament, cementum, and alveolar bone on the facial aspect of the canine teeth were removed, and cellulose filters were placed over the defects. Histologic examination subsequently showed regeneration of the alveolar bone and new attachment of cementum with inserting periodontal ligament fibers.
Researchers have studied the use of these filters on a human tooth.31 Debridement and scaling/root planing were performed on a mandibular incisor with advanced periodontal disease after the elevation of full-thickness flaps. A cellulose filter was placed to cover the defect and part of the alveolar bone. Histologic examination showed new cementum with inserting collagen fibers after 3 months. Disadvantages of the use of cellulose filters include exfoliation, premature removal, and the need for a second surgical procedure for their removal.
Expanded polytetrafluoroethylene membranes
To date, the majority of studies related to membrane barriers have involved e-PTFE membranes. Widely used in many animal and human studies, these membranes have been considered the gold standard against which other types of membranes are compared.2,24 E-PTFE membranes are composed of a matrix of polytetrafluoroethylene (PTFE) nodes and fibrils in a microstructure that varies in porosity and that addresses the clinical and biologic requirements of its intended applications. E-PTFE is known for its inertness and tissue compatibility.32 Its porous microstructures allow the ingrowth and attachment of connective tissue for stabilization of the healing wound complex and the inhibition of epithelial migration. In addition, e-PTFE has a history of safe and effective use as an implantable medical material.18
E-PTFE membrane barriers consist of two parts. The first is a coronal border (open microstructure collar) that facilitates early clot formation and collagen fiber penetration to immobilize the membrane (Fig 3-4). The collar may also stop apical proliferation of epithelium through a phenomenon called contact inhibition. The second part is an occlusive portion that prevents gingival tissues outside the barrier from interfacing with the healing process at the defect site.2,20 Two different configurations of e-PTFE membranes can be used according to the situation. The transgingival design is used to treat defects associated with structures, such as teeth, that extend through the gingiva. The submerged design is used to treat situations, such as bony defects, where there is no communication with the oral environment.32
Titanium-reinforced e-PTFE membranes were designed to increase the tent-like effect that is needed when the defect morphology does not create an adequate space.33 Space creation and maintenance have been recognized as important prerequisites for achieving regeneration (Fig 3-5). Space making is also dependent on the mechanical ability of a membrane to resist collapse. The first membranes created for regeneration were intended to possess a certain grade of stiffness. However, the membranes had a degree of memory that limited their use to situations in which adequate membrane support would be provided by the adjacent bone.18 Therefore, titanium-reinforced e-PTFE membranes were created for use in situations where the anatomy of the defect may cause nonreinforced material to collapse into the defect space or where more space is needed for the desired regeneration. Like traditional e-PTFE membranes, titanium-reinforced membranes are available in transgingival and submerged configurations.32, 34 Several studies have shown titanium-reinforced e-PTFE membranes to have substantial biologic potential for the regeneration of alveolar bone and periodontal structures. The space that was created was more predictable and resistant to collapse due to overlying mucosal tissue compared with the space that was created with nonreinforced membranes (Fig 3-6).35, 36
Gore-Tex membranes are available for a variety of medical applications. This hollow, tube-shaped configuration is 12 inches long and 0.5 inches in diameter and is designed for vascular repair.
This nonresorbable e-PTFE membrane (Gore-Tex) in its submerged configuration is designed for use with defects that can be completely isolated from the oral environment by means of flap repositioning. The periphery of the membrane has an open microstructure that permits early clot formation and collagen fiber penetration to help stabilize the membrane. The open microstructure also stops apical proliferation of epithelium by contact inhibition. The occlusive center portion prevents gingival tissue invasion.
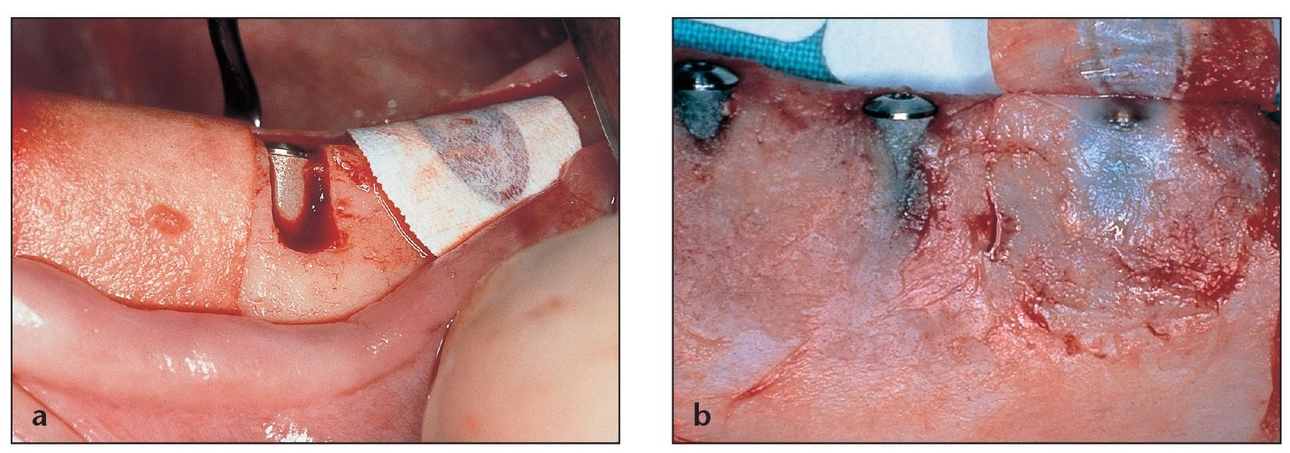
(a) Because the size of the defect in this anterior mandible site (from tooth loss due to trauma) prevents membrane collapse, an e-PTFE membrane without titanium reinforcements is indicated. Implants are placed simultaneously. One fixation screw is placed under the membrane to “tent up” the membrane. Additional screws will be used to fixate the membrane.
(b) Note the excellent ridge volume at membrane removal. The membrane was submerged, allowing for good closure and isolation to maintain the marginal tissues, as shown by the papilla.
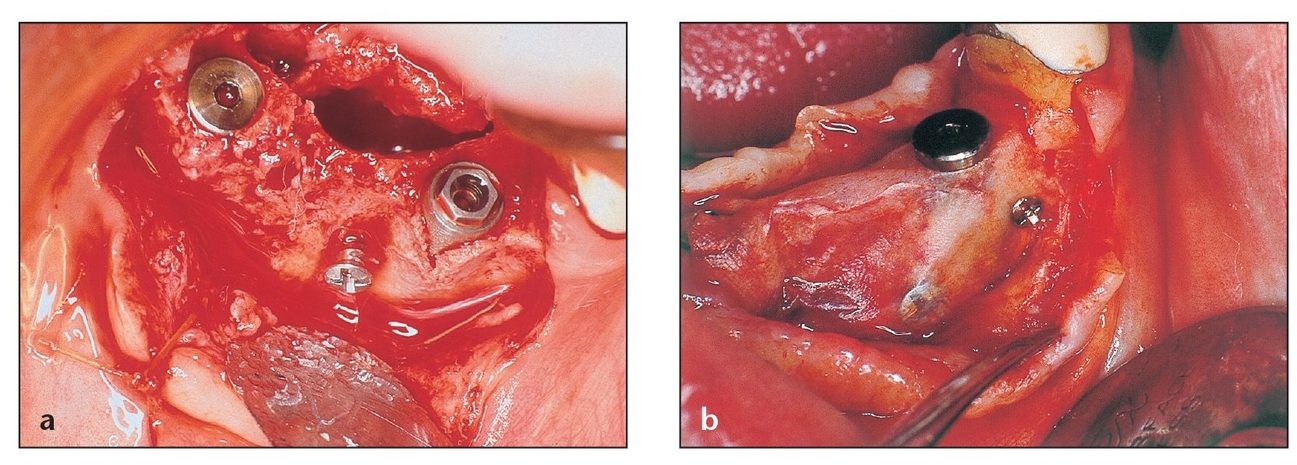
(a) This e-PTFE membrane configuration contains transversing titanium bands designed to create a noncollapsible space for healing.
(b) This transgingival nonresorbable e-PTFE membrane configuration, which contains titanium bands, is used for a defect that extends through the gingiva into the oral cavity.
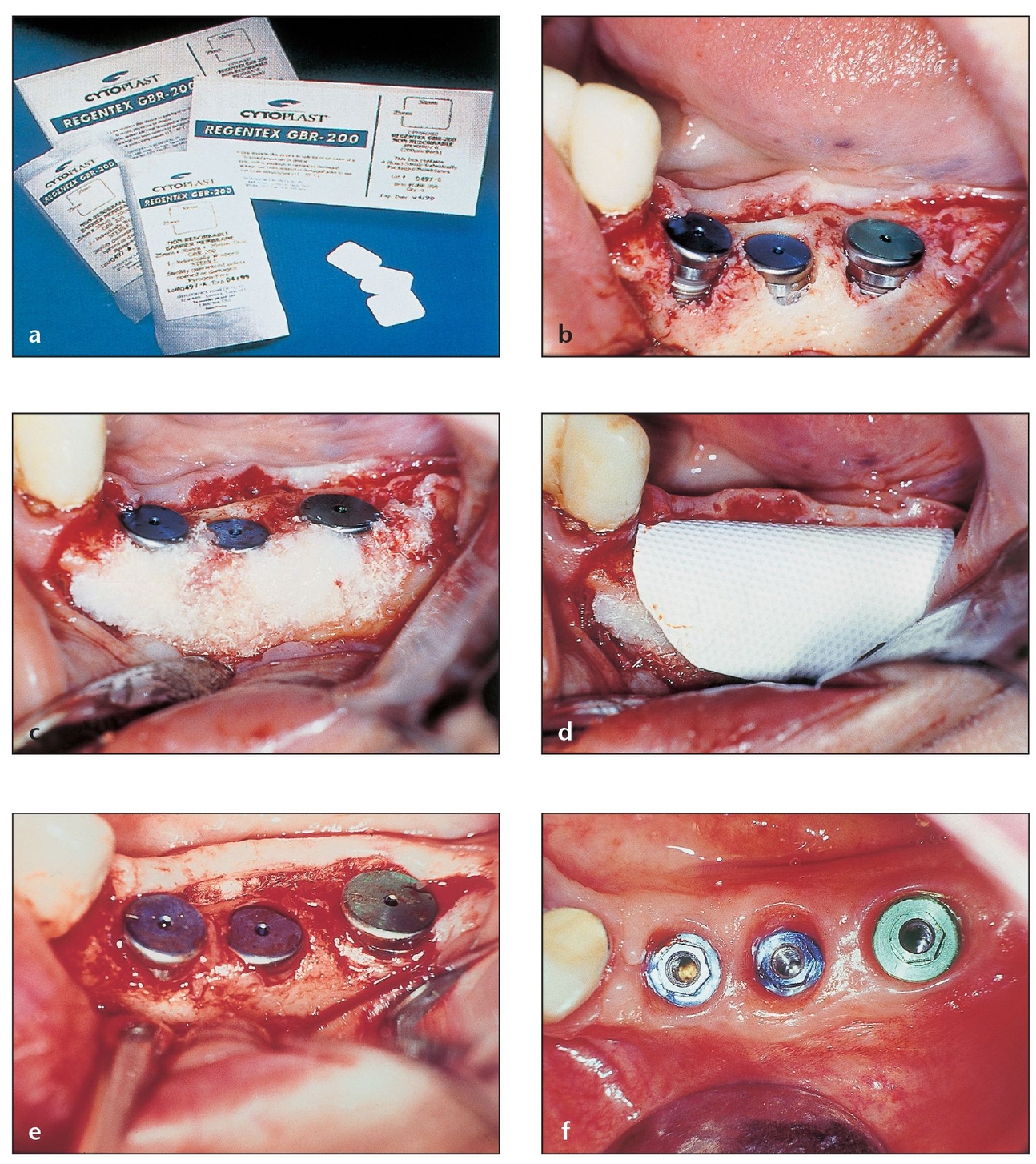
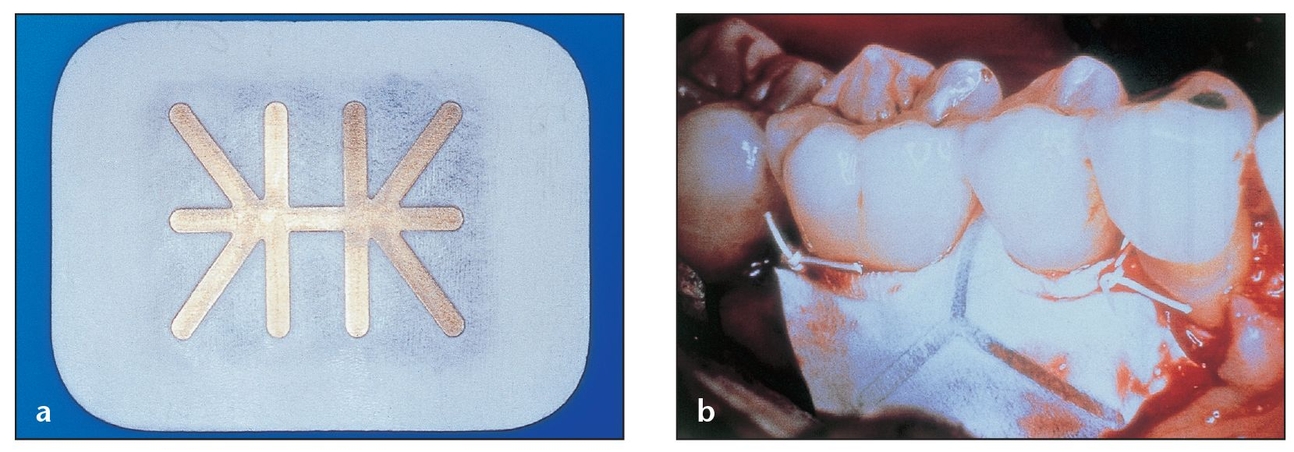
Fig 3-7 Nonexpanded, high-density PTFE membranes have shown promise as effective barriers that allow bone deposition in osseous defects, but further studies are needed to evaluate their effect.
(a) Regentex GBR-200, made of nonexpanded PTFE, is purportedly designed to be used as a nonresorbable membrane barrier.
(b) A defect in the bone leaves three implants slightly exposed on the buccal side.
(c) Covering the defect with a graft does not guarantee graft dispersal or that undesirable soft tissue will not migrate and fill the defect.
(d) Membrane is cut to an appropriate size to cover the grafted site.
(e) Optimal bone coverage means that the membrane helps contain the particulate graft, encouraging bony tissue regeneration.
(f) Good hard and soft tissue contours 15 days after implant exposure demonstrate the value of using bone graft and a barrier membrane.
The main disadvantage of the use of e-PTFE membranes is that a second surgical procedure is required for their removal, which increases the cost and surgical trauma to the patient.2 With the use of these membranes, clinicians can control the length of time that the membrane remains in place. It has been suggested that healing times may vary among different types or sizes of defects, especially bony defects of the alveolar ridge.18,36 The principal advantage of the use of this membrane is that it retains its functional characteristics long enough for adequate healing to occur and then it can be eliminated immediately. After removal, there is no possibility of breakdown products interfering with the maturation of the regenerated tissues.24
In some situations, nonresorbable membranes provide a more predictable performance, with less risk for long-term complications and with simplified clinical management.18 The use of e-PTFE membranes may be advantageous in situations where soft tissue management problems are anticipated and complete flap closure cannot be achieved. If premature removal of the membrane is required, it can be accomplished without interfering with the regenerated tissues.24
The use of a nonexpanded, high-density PTFE barrier (Regentex GBR-200, Oral-tronics, Bremen, Germany) for barrier membrane techniques has also been evaluated. 25 The membrane appeared to be well tolerated by the soft tissue, caused no inflammation or drainage, and provided an effective barrier that allowed bone deposition in the osseous defects (Fig 3-7). However, additional clinical studies are needed to evaluate the effect of this type of membrane.25 The purported advantage of its use is that it can be left exposed in the oral cavity without risk of compromising the bone regeneration process (Fig 3-8).
Dental rubber dam
A number of studies have suggested the suitability of using dental rubber dam as a barrier membrane for GTR in periodontal procedures.37, 38, 39, 40, 41 For example, a 1994 study documented five cases in which rubber dam was used as a barrier in the GTR treatment of infrabony defects.37 According to this study, the barrier was placed subsequent to flap elevation, debridement, and root planing. Rubber dam covered the defect and surrounding bone; the surgical flap covered rubber dam, which was removed after 5 weeks. Clinical measurements and a reentry procedure at 1 year showed the suitability of the rubber dam barrier. A 1998 study concluded that dental rubber dam can be used as a barrier membrane for GTR procedures but that e-PTFE membranes provide better probing, attachment level, and vertical bone gains. This is probably because of rubber dam’s inability to cover the regenerated tissue completely as a result of consistent recession of gingival tissues in dam-treated sites.40 A 2002 study compared the connective tissue and bacterial deposits on rubber dam sheets and e-PTFE membranes used as barrier membranes in GTR and found no significant difference in the total number of connective tissues on both types of membranes.41 In fact, the total amount of bacteria on rubber dam sheets was statistically lower than the amount on the e-PTFE membranes. The comparability in the number of connective tissues found on both types of membranes suggests the suitability of using rubber dam sheets as barrier membranes in GTR. These results reflect the conclusions of earlier studies that suggested no significant healing differences between rubber dam and e-PTFE membranes.38,39
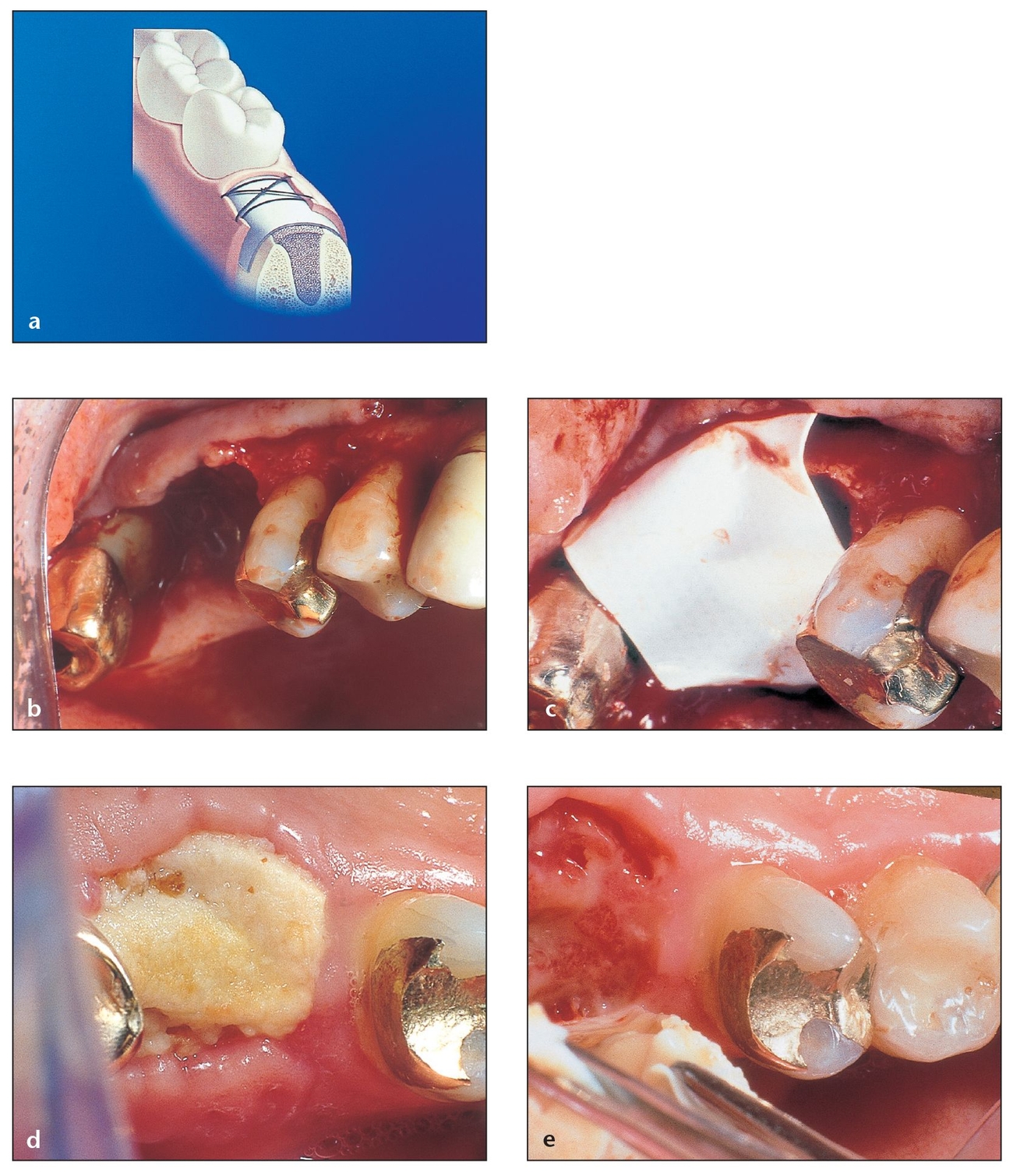
Fig 3-8 Nonexpanded, high-density PTFE membranes have the purported advantage of permitting exposure to the oral cavity without risk of compromising the bone regeneration process.
(a) The Regentex GBR-200 membrane permits exposure while preventing bacteria from seeping into the defect.
(b) A large defect after tooth extraction requires the use of a barrier membrane to retain grafting material and to prevent epithelial tissues from growing into the socket. Because of this, primary closure will be difficult. The socket is grafted with bone graft material.
(c) A Regentex membrane is then placed to extend a few millimeters beyond the defect margins.
(d) Despite the lack of complete closure, gingival epithelium did not enter the socket but instead migrated over the site. The layer of yellowish plaque that formed over the membrane can be easily removed. The absence of inflammation suggests that no infection has occurred.
(e) After the membrane is removed, the epithelium that formed over the socket and under the membrane can be seen. Beneath this tissue, the desired osteoid tissue continues to mature.
Titanium membranes
Titanium membranes can also be used for GTR/GBR in oral implant applications. These membranes are totally inert and osteophyllic. It has been reported that in a series of 42 patients using a 22-µm-thick titanium membrane in conjunction with composite grafting of autologous bone and demineralized freeze-dried bone for treatment of peri-implant bony defects at the time of implant placement, satisfactory augmentation was achieved in 90% of cases, surpassing the success rate of Gore-Tex membranes.42
(a) Three defect areas in the same alveolar ridge, similar in extension and configuration, were left to heal under different conditions: one covered with a resorbable membrane (left), the second covered with a nonresorbable membrane (right), and the third left uncovered as a control (center).
(b) Different levels of bone formation can be observed at each site. The site covered with the nonresorbable membrane shows the greatest amount of bone formation.
Resorbable Materials and Devices
The main advantage of using resorbable membranes is the avoidance of a second surgical procedure, thus reducing patient morbidity and expense.26 A disadvantage is that material exposure or flap dehiscence can cause postoperative tissue management problems. Material exposure after surgery can lead to bacterial growth, alteration of fibroblast morphology, and migration, all of which may jeopardize the success of the regeneration process. Another common problem is the difficulty of preventing membrane collapse into the defect, which can result in inadequate space making.43
The use of resorbable barriers is based on criteria similar to those for nonresorbable membranes, and the degradation process should not negatively affect the regenerative outcome (Fig 3-9).2,18 Resorb-ability may be associated with degradation through enzymatic activity (biodegradation) or hydrolyzation (bioabsorption) as a cellular response from the surrounding tissue. The inflammatory response should be minimal and reversible and must not interfere with regeneration.2 A large number of resorbable barrier materials exist, some more popular than others.
Collagen membranes
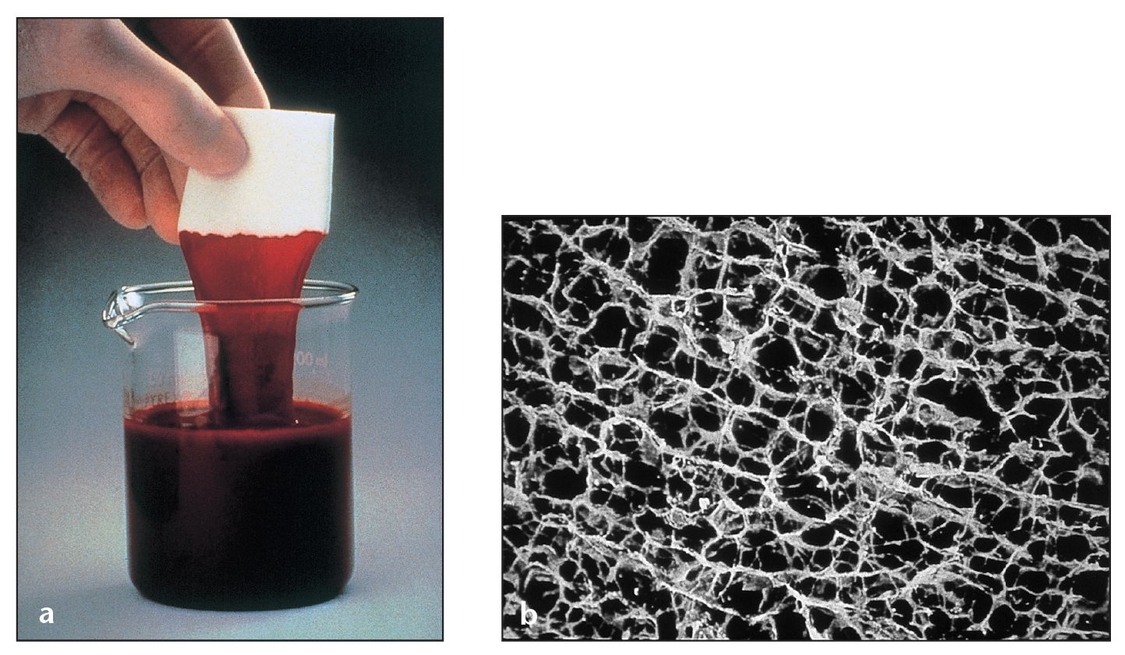
Collagen is a physiologically metabolized macromolecule of the periodontal connective tissue that has two different properties: chemotaxis (for fibroblasts) and hemostasis. It is also a weak immunogen and may act as a scaffold for migrating cells (Fig 3-10).26, 44 Collagen possesses several characteristics that make it a suitable barrier material, including favorable effects on coagulation and wound healing, controlled cross-linking, low antigenicity and extensibility, high tensile strength, and fiber orientation. Collagen can also be produced in various forms such as sheets, gels, tubes, powders, and sponges (Fig 3-11).45
Since the mid-1990s, several collagen-based materials have been used as a barrier membrane in periodontal and oral and maxillofacial surgery. Processed bovine type I collagen membranes, originating both from tendons (Fig 3-12) and from dermal sites, have been evaluated for barrier membrane procedures in animals and humans with positive results.26 Multicenter studies have shown results that are equivalent to those obtained using nonresorbable membranes in periodontal defects. 26
Early studies established the potential for reabsorption of collagen membranes. 46, 47 Results were limited because of rapid degradation (30 days) caused by enzymes found in plaque and healing wounds. Because of these findings, researchers improved the quality of collagen membranes through the use of bilayered barriers to compensate for the premature degradation of the external barrier and by adding heparin sulfate and fibronectin to the internal barrier. Fibronectin acts as a chemotactic factor for fibroblasts and is able to bind the heparin sulfate to the collagen membrane. The inner barrier is designed to act as a second barrier for the migrating epithelium and to serve as a delivery system for fibronectin and heparin sulfate. The results of this study demonstrated that the enriched collagen barrier had improved properties to retard apical migration of epithelium compared with nonenriched membranes.48
(a) A collagen membrane such as CollaTape (Zimmer Dental, Carlsbad, CA) is an ideal carrier for substances such as antibiotics or platelet-rich plasma (PRP). Because collagen enhances platelet aggregation, this membrane helps stabilize blood clots.
(b) More than 90% of the sponge-like surface of CollaTape (a collagen material obtained from bovine Achilles tendon) consists of open pores that retain fluids.

Absorbable collagen dressings are available in different configurations and sizes. CollaTape (center ) can be used to cover and stabilize graft materials, CollaPlug (Zimmer Dental; left) can be placed into or over extraction sockets, and CollaCote (Zimmer Dental; right) can be used to fill in harvested soft tissue sites, such as the palate.
A multicenter study that evaluated the use of bovine tendon type I collagen membranes for membrane barrier procedures in human Class II furcation defects compared the efficacy of bioabsorbable collagen membranes with that of surgical debridement or e-PTFE membranes.26 The results of the study showed that collagen membranes were clinically effective and safe for use in periodontal barrier membrane procedures. The gain in attachment using collagen membranes was equal to or greater than that obtained with the use of surgical debridement or e-PTFE membranes.
Fig 3-12 Processed bovine type I collagen membranes have shown results equivalent to those obtained using nonresorbable membranes in periodontal defects.
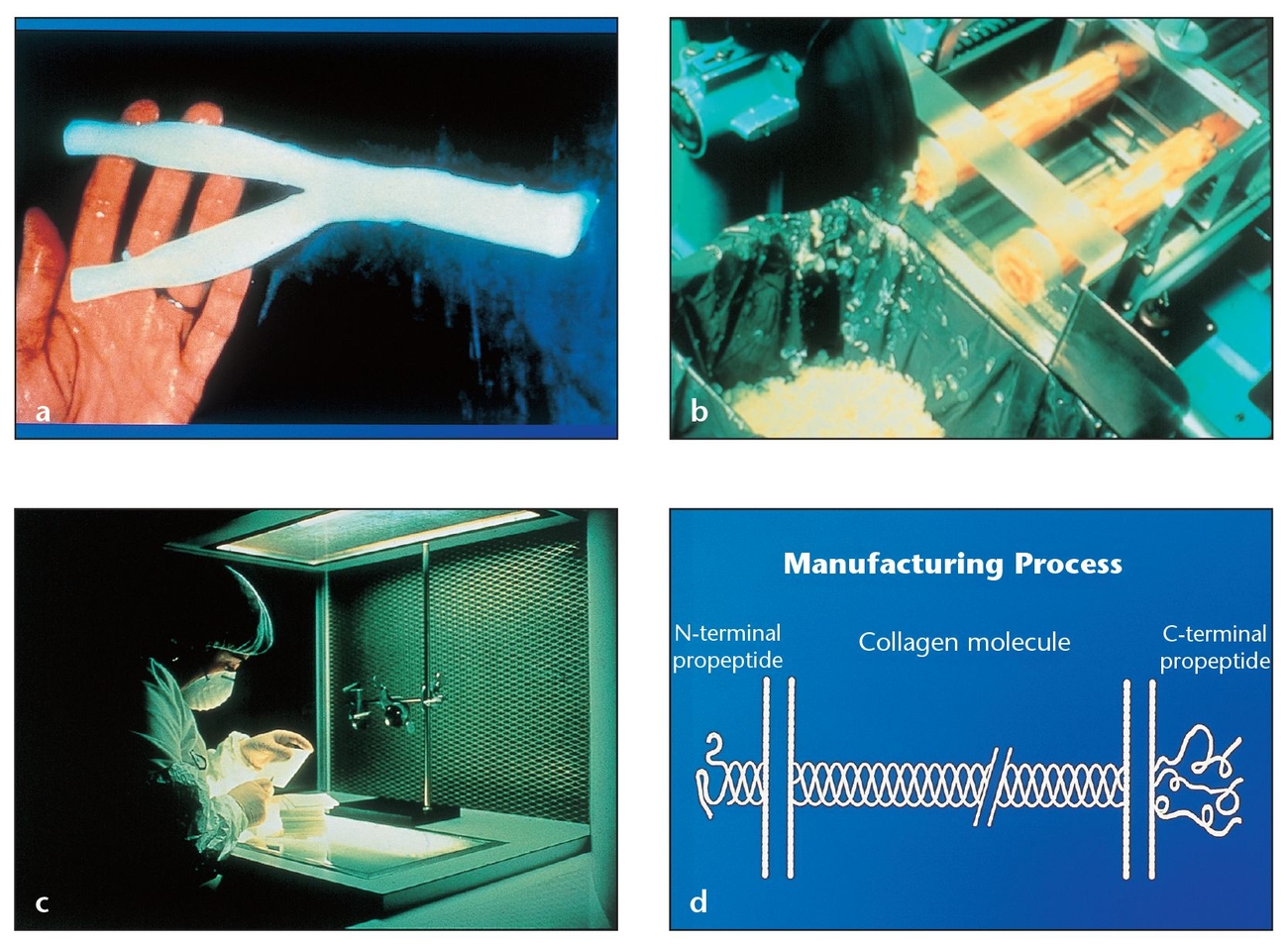
(a) Bovine deep flexor (Achilles) tendon is the type I collagen source for the BioMend (Zimmer Dental) resorbable membrane.
(b) Careful processing of the tendon includes the removal of antigenic portions.
(c) The entire manufacturing process involves rigorous inspection of the material.
(d) The removal of antigenic portions of the polypeptide chain increases the biocompatibility and recipient tolerance of the BioMend membrane.
Another study showed successful treatment of one-, two-, and three-wall defects via a collagen membrane combined with antigen-extracted allogeneic bone and collagen gel.49 In this study, a 1- to 2-mm film of collagen gel was placed in the base of the defect, the allogeneic bone was packed into the defect, and a collagen membrane was placed over the defect. Another study compared e-PTFE membranes with type I collagen in GTR of Class II mandibular molar furcation defects; healing intervals were 1 year, and clinical assessments took place at 8 months. Reentry occurred at 1 year.50 The researchers observed no significant differences between the two types of membranes with regard to reduction of pocket depth, attachment gain, or horizontal defect filling. The collagen membrane outperformed the e-PTFE membrane with regard to vertical fill.
The advantages of collagen membrane use include minimal postoperative complications, a good healing rate, and no incidence of material dehiscence, tissue perforation, sensitivity reactions, immune response, tissue sloughing, delayed healing, or postoperative infection.51 Collagen appears to be a useful and beneficial membrane material for regenerative therapy because collagen membranes meet the criteria for membrane barrier techniques—space creation, tissue integration, cell oc-clusivity, biocompatibility, and clinical manageability.51
One collagen product, CollaTape, has been used for minor oral wounds, to close graft sites, and to repair schneiderian membranes. Its benefits include controlling bleeding and stabilizing blood clots, protecting wound beds, and providing a matrix for tissue ingrowth associated with GTR. It fully resorbs in 10 to 14 days. Another product, Paroguide (Coletica, Lyon, France), is made of cross-linked bovine collagen from calfskin. It is composed of 96% type I collagen and 4% chondroitin-4-sulfate and has a resorption rate of 4 to 8 weeks.52 Paroguide is an opaque, off-white substance of medium rigidity. It comes packaged in two double-chambered blister packs, one of which holds the membrane, the other of which contains two modeling membranes used to fashion models for the actual membrane to be placed. Paroguide can be used to reconstruct osseous defects, to repair bone furcations, to augment the alveolar ridge, and to cover peri-implantation spaces and extraction sites. Resorbable sutures can be used to hold the membrane in place.
BioMend is an absorbable collagen membrane derived from bovine Achilles tendon that has been shown to be effective in bone regeneration procedures.53 An in vitro study concluded that the BioMend membrane enhanced early osteoblast (MC3T3-E1) attachment.30
BioMend is a compressed, nonfriable type I collagen matrix. Paper-white when dry and leather-like in its surface texture, its condensed composition of laminated sheets can be seen in cross section. When wet, the membrane becomes translucent, but it is not slippery and can be adapted to the tooth structure. Antigenic portions of the collagen molecule are removed when the product is manufactured, increasing its biocompatibility and tissue tolerance. Its immune response has been clinically verified, and separate production lots are verified as nonpyrogenic.53 BioMend resorbs into gingival connective tissue via enzyme (collagenase) degradation. It is designed to remain intact for 4 weeks and has an average life span of 6 to 7 weeks. Full resorption takes place within 8 weeks (Fig 3-13).
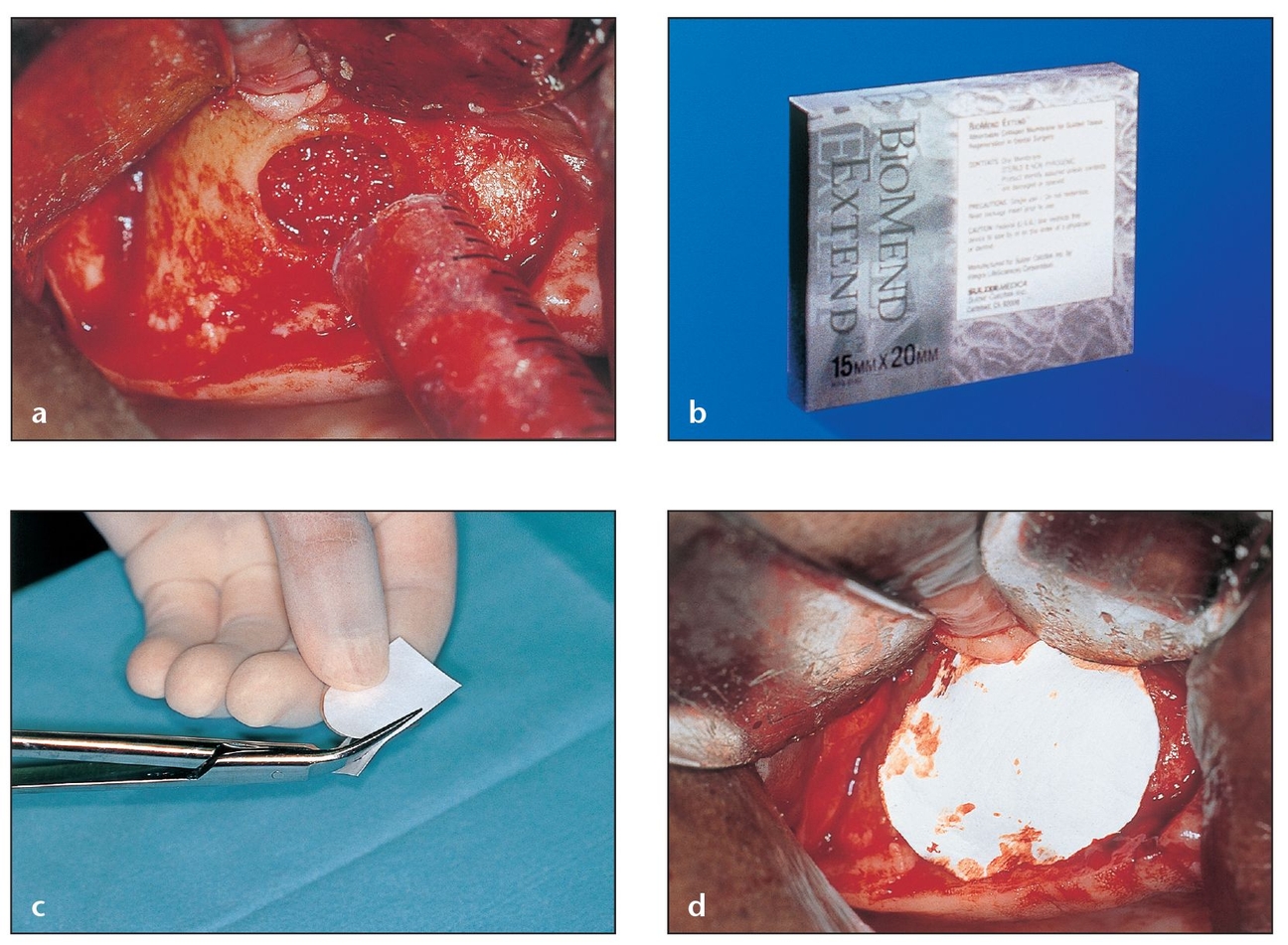
Results from eight centers and 133 patients compared BioMend absorbable collagen membranes with e-PTFE membranes or surgical debridement used to treat furcation defects.54 A statistically significant decrease in probing depth and clinical gain in probing attachment were noted in patients treated with BioMend versus e-PTFE membranes. Only the BioMend membrane patients experienced complete furcation closures. BioMend Extend is a longer-lasting version of BioMend that is thicker, more pliable, and tear-resistant. It is resorbed within 18 weeks and thus able to maintain a regenerative barrier for a longer period (Fig 3-14).
Ossix (ColBar R&D, Herzliya, Israel) is a resorbable collagen membrane that functions as a regenerative barrier for 6 months after placement. Its durability addresses a problem associated with successful bone regeneration using collagen membranes—namely, the degradation of such membranes by mammalian collagenase when submerged or by bacterial collagenase when exposed.55 A group of researchers explored the barrier membrane potential of Ossix for bone augmentation.56 For clinical purposes, both primary and, in particular, secondary healing patterns were studied. Researchers documented soft tissue healing photographically; image analysis on digitized photographs was then used to calculate the size of dehiscences. Researchers dissected and histologically evaluated the barrier remnants during reentry. The study indicated that the mean value for dehiscences was 35.5 mm; after evidence of exposure, all dehiscences healed within 4 weeks. Statistically significant differences were noted between weeks 2 and 6 for previously exposed sites. Histologically, the study showed direct apposition of fibrous and bone tissues on the membrane surface. The researchers concluded that when the membrane was exposed, gingival dehiscences always disappeared in subsequent weeks with no effect on healing. Bone regeneration occurred during barrier stability over 6 months, according to the histologic results (Fig 3-15).
(a) The BioMend membrane is packaged in an envelope with templates to accommodate defects of different sizes and shapes.
(b) Under a scanning electron microscope, the BioMend membrane appears as a condensed laminated sheet in cross-section (200× magnification).
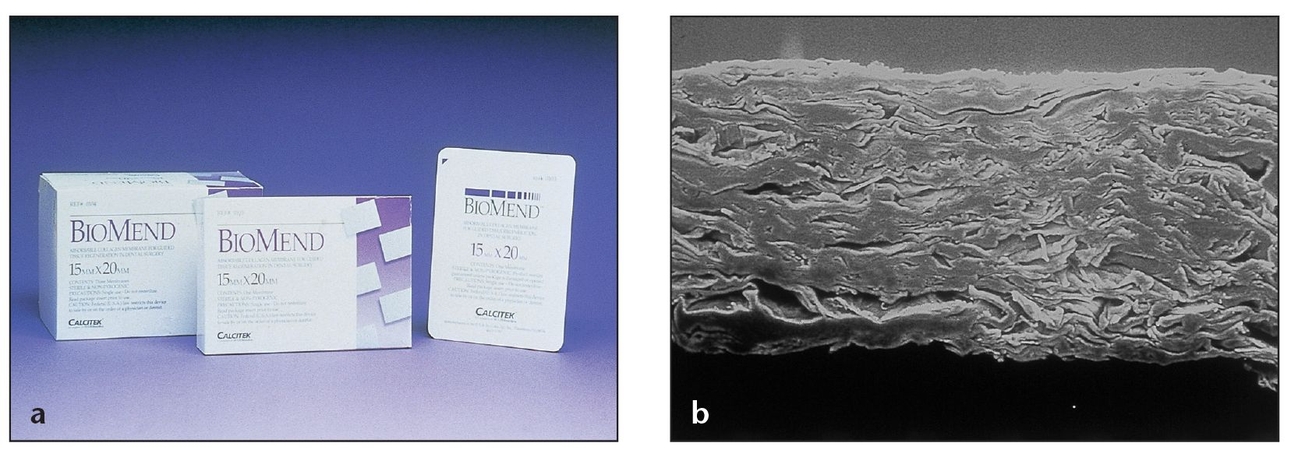
Fig 3-14 BioMend Extend, a longer-lasting absorbable collagen membrane, is fully absorbed within 18 weeks and maintains a regenerative barrier for a longer period of time.
(a) A sinus cavity window that has been fully grafted during a sinus lift is covered with a barrier membrane to protect and isolate the graft from epithelial downgrowth.
(b) The pore size (0.004 µm) of the BioMend Extend membrane, which resorbs more slowly than BioMend, effectively retards epithelial invasion during early healing phases.
(c) The BioMend Extend membrane is rigid enough to be trimmed to the desired shape.
(d) The material’s stiffness makes it ideal for the semi-flat configuration over the sinus window. The membrane should be trimmed 2 to 3 mm beyond the periphery of the window.
Another study compared qualitative histologic results from the use of Ossix and deproteinized bovine bone mineral (DBBM) as a space maintainer versus the standard e-PTFE membrane (Gore-Tex) and the same bone substitute, with the latter used as the control group.57 According to the Mann-Whitney test, differences in results were not statistically significant. When barrier exposure occurred, it did not interfere with the histologic outcome in either group. Thus, bone regeneration results were comparable for Ossix and the e-PTFE barrier.
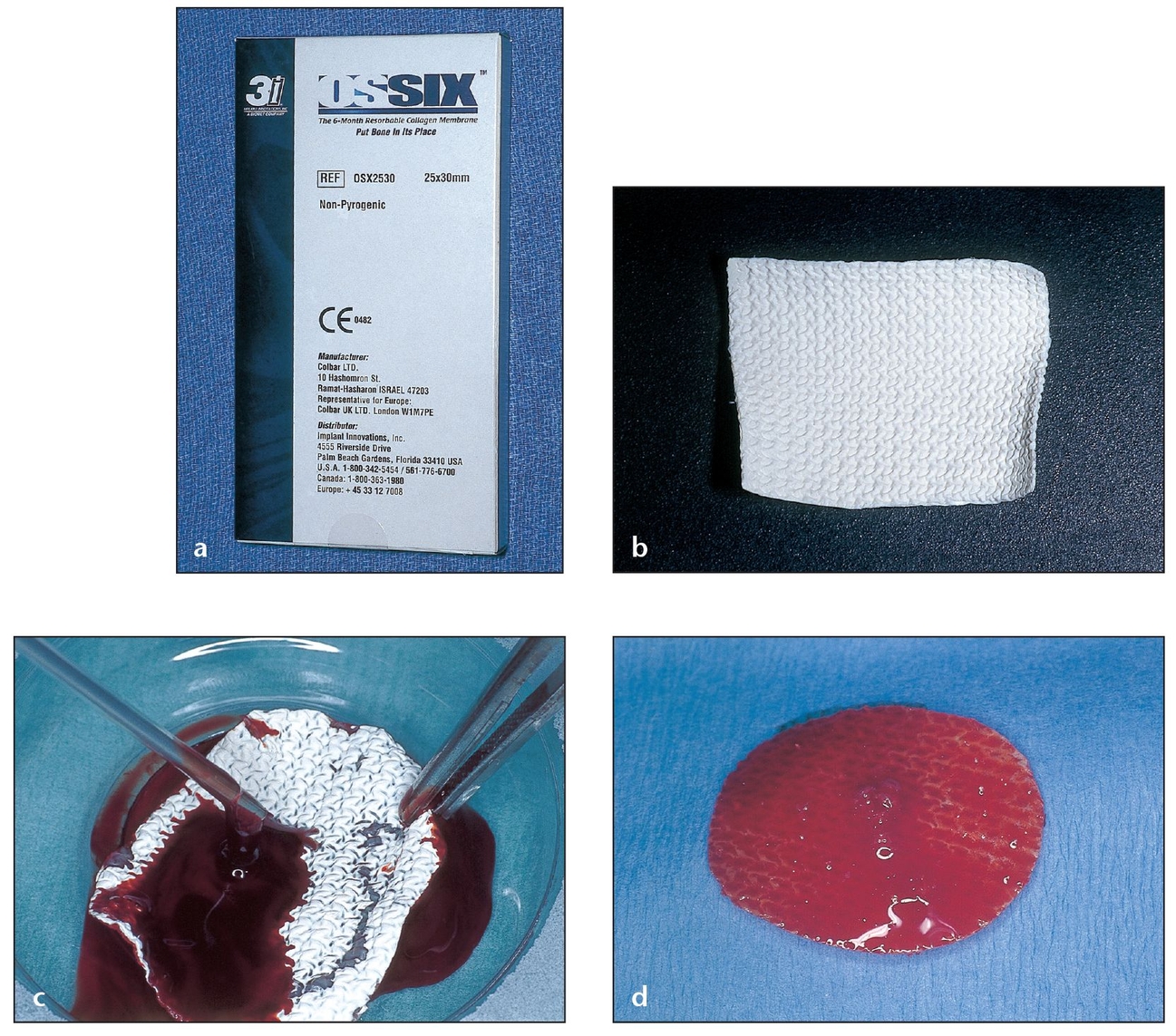
Fig 3-15 Ossix, a resorbable collagen membrane, can remain for up to 6 months after placement.
(a) The collagen in the Ossix membrane is cross-linked with glucose metabolites to help reduce inflammation and resist absorption even after exposure.
(b) Easy to cut and shape, this collagen membrane’s flexibility helps it adhere to tissues during application without tacking or suturing.
(c) PRP growth factors will enhance the function of the collagen membrane; the PRP easily adheres to both sides of the membrane. The Ossix membrane remains manageable even after wetting.
(d) An Ossix membrane saturated with PRP for the recipient site will retain its malleability.
Bio-Gide (Geistlich Biomaterials, Wohl-husen, Switzerland) is a slow-resorbing (taking at least 4 months), pure (no organic residue or additional chemicals) bilayer collagen membrane composed of porcine collagen types I and III. Any possibility of viral or bacterial contamination has been eliminated from the produ/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


