28
Renal Function Tests, Renal Disease, and Dialysis: Assessment, Analysis, and Associated Dental Management Guidelines
KIDNEY FUNCTION TESTS
Tests evaluated to assess kidney function are:
Serum Creatinine (S.Cr)
Creatinine is a waste product generated from muscle cell breakdown, and it is filtered out in the urine by the kidneys. As kidney function decreases, the serum creatinine (s.Cr) levels rise. Muscle mass and diet can affect serum creatinine values, so it is best to estimate renal function by calculating the GFR. The normal s. creatinine in most labs is 0.4–1.2 mg/dL. In general and oversimplified terms (given the variability of s. creatinine based on muscle mass), a patient is said to have a 50% reduction of kidney function when the serum creatinine is ≥1.7 mg/dL in men and ≥1.4 mg/dL in women.
It is safe to assume that individuals with a serum creatinine of 2.0 mg/dL have moderate-to-severe decrease in GFR, regardless of the equation used to estimate GFR.
Creatinine Clearance (CrCl)
The creatinine clearance shows how well the kidneys are functioning. This test indicates how efficiently the kidneys can remove creatinine from the blood and pass it into the urine. The test compares the amount of urine creatinine in a 24-hour collection with the level of serum creatinine.
Cockcroft-Gault (C-G) Creatinine Clearance Equation
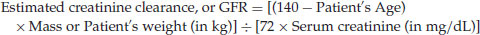
If the patient is female, the clinician must multiply the resulting value by 0.85. Creatinine clearance is measured as mL/min. The normal creatinine clearance is 80–130 mL/min.
Proteinuria
Another marker of kidney function is the presence of protein in the urine. Protein normally does not filter out of the kidneys. Healthy kidneys remove all wastes from the blood but do not remove protein. Diseased kidneys may fail to separate albumin from the wastes. Initially, only small amounts of albumin leak into the urine. This microalbuminuria is an indication of deteriorating kidney function. As kidney function worsens, the amount of albumin and other proteins in the urine increases, resulting in proteinuria. A 24-hour urine collection measures the total amount of protein lost in the urine.
Blood Urea Nitrogen (BUN)
Urea is formed in the liver as a waste product when protein is broken down in the body. The urea is then eliminated in the urine. Blood urea nitrogen (BUN) measures the amount of nitrogen in the blood. The nitrogen comes from urea. BUN thus gives an estimate of how effectively the kidneys are in removing urea from the blood. The normal BUN in most labs is 7–20 mg/dL.
Glomerular Filtration Rate (GFR)
The GFR shows how efficiently the kidneys are filtering wastes from the blood in normal and diseased patients. GFR is estimated by using the modification of diet in renal disease study group (MDRD) equation, which is based on the patient’s age, weight, gender, race, and serum creatinine. Use the following site to calculate your patient’s GFR: www.kidney.org/professionals/kdoqi/gfr_calculator.cfm
Serum creatinine-based estimation of GFR provides a basis for the classification of chronic kidney disease.
Glomerular Filtration Rate and Staging of Chronic Renal Disease
The Kidney Disease Outcomes Quality Initiative (K/DOQI) of the National Kidney Foundation (NKF) defines chronic kidney disease (CKD) as either kidney damage or a decreased glomerular filtration rate (GFR) of less than 60mL/min/1.73m2 for three or more months. Kidney damage is defined as pathologic abnormalities or markers of damage, including abnormalities in blood or urine tests or imaging studies. Per K/DOQI, these are the classification stages of CKD:
Patients with CKD stages 1–3 may be asymptomatic. Clinical manifestations typically appear in stages 4–5, but may appear as early as stage 3. A patient is said to have CKD when the GFR is <60 mL/min/1.73 m2 for three months. As a conscientious provider, you must help prevent the progression of kidney disease by using the appropriate AAAs and insist that the patient control associated diseases, such as hypertension and diabetes, which commonly cause CKD.
Renal Imaging
Ultrasound, computed tomography (CT scan), and magnetic resonance imaging (MRI) are tools used to detect unusual changes in the kidney structure or impairment in urinary flow.
CHRONIC KIDNEY DISEASE
Sound knowledge of normal renal physiology is needed to better understand the changes associated with renal dysfunction. The dental provider has to address these alterations before proceeding with dentistry. Additionally, the provider needs to modify the use of anesthetics, analgesics, antibiotics, and antivirals in the dental setting, so the renal-compromised patient can be optimally treated.
Chronic Kidney Disease Pathophysiology
Introduction
The kidney has an innate ability to maintain GFR, even in the presence of injury, via hyperfiltration and compensatory hypertrophy of the remaining healthy nephrons. This nephron adaptability allows for continued normal clearance of plasma solutes. Plasma levels of urea and creatinine start to show significant increases only after total GFR has decreased to 50% when the renal reserve has been exhausted. The plasma creatinine value approximately doubles with a 50% reduction in GFR. A rise in plasma creatinine from a baseline value of 0.6–1.2mg/dL in a patient, although still within the reference range, actually represents a loss of 50% of functioning nephron mass.
Hyperkalemia
CKD patients are able to maintain potassium (K) excretion at near-normal levels as long as both aldosterone secretion and distal renal flow are maintained. Through the effect of aldosterone, the body is also able to increase potassium excretion in the gastrointestinal (GI) tract. Hyperkalemia usually develops when the GFR falls below 20–25mL/min, when the renal excretion of potassium decreases. It can occur sooner in patients who ingest NSAIDS.
Metabolic Acidosis
It is necessary to discuss the normal acid-base balance first and then discuss metabolic acidosis associated with CKD. It is important to note that in the presence of acid-base abnormality, the system that is not primarily responsible for the acid-base imbalance assumes the responsibility for returning the pH to the normal range. Acid-base imbalance can be from multiple causes but the two main systems that can affect the acid-base status are the respiratory system and the renal system. In primary respiratory disorders, the pH and PaCO2 go in opposite directions; in metabolic disorders the pH and PaCO2 go in the same direction.
ABG pattern in metabolic acidosis: pH: <7.35; PaCO2: normal; HCO3: <22mEq/L. The acidosis is less likely to be of respiratory origin and very likely to be of non-respiratory or metabolic origin. A low pH (<7.35) and a low HCO3 (<22mEq/L), which are consistent with acidosis, are very likely due to non-respiratory causes. Common causes of metabolic acidosis include diabetes, shock, and renal failure.
Metabolic alkalosis is not associated with kidney disease, but if the reader is wondering what metabolic alkalosis is, here is a synopsis: Metabolic alkalosis is characterized by an increased pH and increased HCO3 and is seen with hypokalemia, chronic vomiting or diarrhea, or sodium bicarbonate overdose.
Metabolic Acidosis Associated with CKD
In CKD patients, the kidneys are unable to produce enough ammonia in the proximal tubules to excrete the endogenous acid into the urine in the form of ammonium. This accounts for the accumulation of phosphates, sulfates, and other organic anions causing an increase in the anion gap in the stage-5 CKD patient. Metabolic acidosis is associated with protein-energy malnutrition, loss of lean body mass, and muscle weakness.
Metabolic acidosis plays a role in the development of renal osteodystrophy, as bone acts as a buffer for excess acid, resulting in mineral loss. Acidosis interferes with vitamin D metabolism, and patients who are persistently more acidotic are more likely to have osteomalacia or low-turnover bone disease.
Refer to Chapter 29 for a discussion on respiratory acidosis-alkalosis, and refer to Chapter 41 for a detailed discussion outlining the role of parathyroid glands, vitamin D, kidney, and gut in normal calcium metabolism.
Salt and Water Retention
Failure of sodium and free water excretion by the diseased kidney generally becomes clinically evident when the GFR falls below 10–15mL/min. This leads to peripheral edema, pulmonary edema, and hypertension.
Anemia
Normochromic normocytic anemia develops from decreased renal synthesis of erythropoietin, the hormone responsible for bone marrow stimulation for red blood cell (RBC) production. It starts early and becomes more severe as the GFR progressively decreases. In the presence of less viable renal mass, no reticulocyte response occurs. RBC survival is decreased, and tendency of bleeding is increased from the uremia-induced platelet dysfunction.
CKD and Calcium Metabolism
CKD results in a fundamental disruption of the normal regulation of extracellular calcium, bone calcium, and vascular calcification. Vitamin D absorbed from sunlight and dietary vitamin D are converted in the liver to calcidiol or 25-hydroxyvitamin D, [25(OH)D]. 25(OH)D is the specific vitamin D metabolite that is measured in the serum to determine a pa/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


