26
Anterior Dental Aesthetics: Bleaching
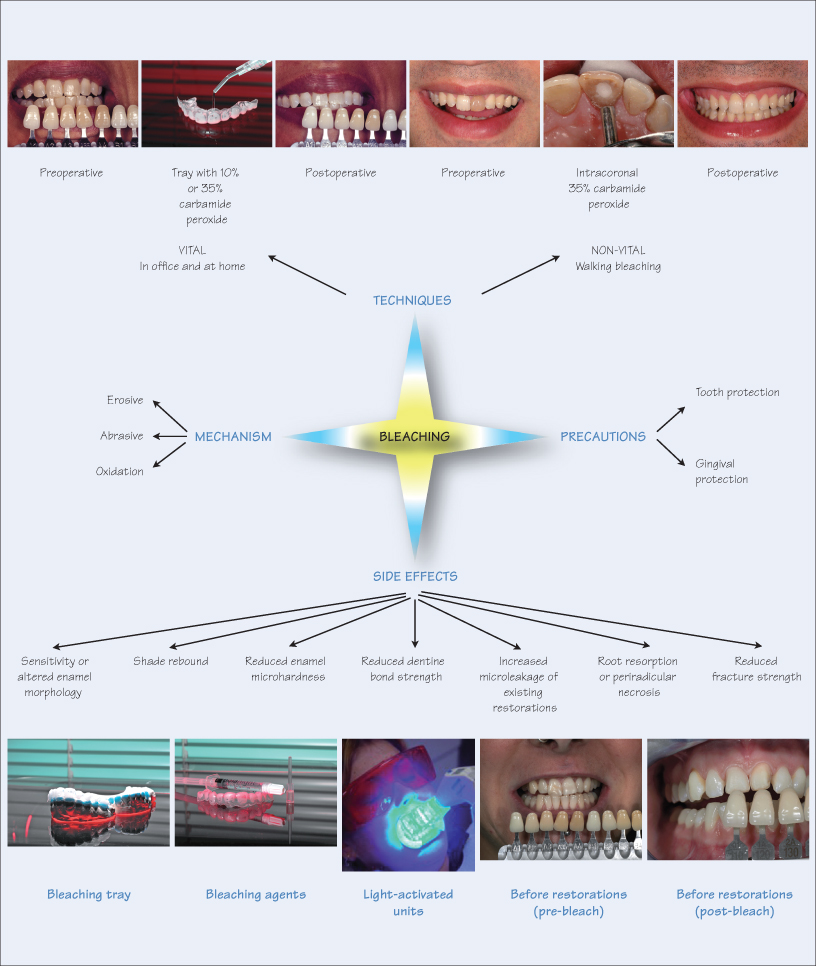
Dental bleaching is an effective, economical and minimally invasive approach to whitening discoloured teeth. Since its introduction two decades ago by Haywood and Haymann, the at-home technique has proved a successful treatment modality for improving unsightly stained teeth. Bleaching is also therapeutic for periodontal health and favours maintenance of oral hygiene. Furthermore, the transient irritation of soft and hard tissues is innocuous and reversible.
Mechanism
Whitening teeth is achieved by any one of three processes, i.e. erosive, abrasive and oxidation. The most popular method is oxidation since erosion and abrasion potentially damage hard tissues by irreversibly altering tooth morphology. Furthermore, excessive enamel loss is counterproductive for tooth whitening, since it allows visibility of the darker underlying dentine.
The oxidation process uses bleaching chemicals that penetrate the interprismatic enamel substrate and convert the pigmented carbon rings to lighter carbon chains. The higher the concentration of the bleaching agent the greater and more rapid the effect. This process alters enamel micromorphology, affecting both the organic and inorganic phase of the hard tissue. The negative effect of oxidation is that bleaching chemicals, with a low pH, demineralise enamel. However, the effect is usually transient, since calcium in the saliva and use of fluoride dentifrices reverse the effect by remineralisation of the enamel surface layer.
Techniques
The commonly used bleaching agents are carbamide peroxide (urea peroxide) in concentrations ranging from 1% to 45%, hydrogen peroxide (3% to 50%) and sodium perborate. These agents are available as gels, varnishes, mouthwashes and toothpastes. The concentration of the bleaching agent determines the duration of treatment. A high concentration results in rapid whitening in a shorter time, while lower concentrations require longer to take effect. There are two main methods for bleaching, either />
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


