chapter 22 Armamentarium
INTRAVENOUS DRUG ADMINISTRATION
Direct Intravenous Administration
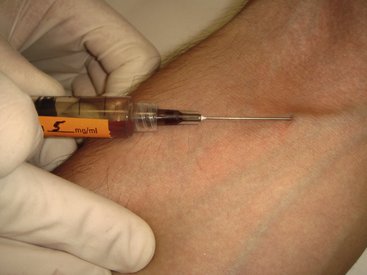
In direct IV administration, a tourniquet is placed on the patient’s arm, engorging the veins, the injection site is prepared, and the needle of the syringe containing the drug(s) is placed into the lumen of the vein, in effect doing the venipuncture with the drug-containing syringe. After ensuring that the needle tip lies within the lumen of the vein (aspiration of blood back into the syringe), the dentist or assistant removes the tourniquet, and the drug is slowly administered into the vein. Following drug administration, the needle is removed from the vein, pressure is applied to the site to stop bleeding, and the planned treatment begun. No access to the vein is maintained during the procedure (Figure 22-1).
Needle Maintained in the Vein Without Continuous Infusion
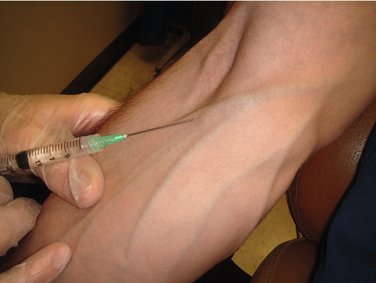
When the needle is maintained in the vein without an infusion, the tourniquet is placed, the veins are engorged, and the tissues are prepared in the usual manner. A winged infusion set or a hollow metal needle is used for venipuncture. Following successful venipuncture, the tourniquet is removed, and the syringe (without a needle attached) is connected to the needle that has been left in the vein and taped into place. After the drug is titrated to effect, the syringe is detached from the needle, and a second syringe containing a solution such as sterile water for injection is attached to the needle. The dental procedure is begun, with the dentist or assistant periodically flushing the needle with 1 ml of solution to keep the vein patent (Figure 22-2).
Continuous Intravenous Infusion
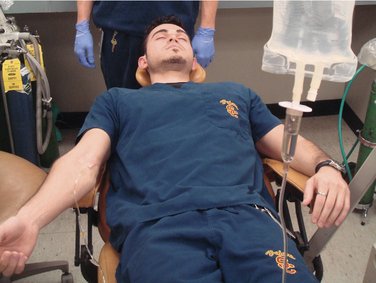
With a continuous IV infusion, an indwelling needle or catheter is attached to a length of tubing that in turn is connected to a bag of IV infusate. The same venipuncture procedure is carried out that was described for the first two techniques. Following removal of the tourniquet, the flow of IV solution is started, and the needle or catheter is secured. The IV drugs are administered through an injection port on the tubing, and the drug syringe is then removed. The rate of the IV infusion is adjusted to maintain a slow flow that will prevent needle occlusion during the dental procedure, which is then begun (Figure 22-3).
ADVANTAGES AND DISADVANTAGES OF VARIOUS METHODS
INTRAVENOUS INFUSION SOLUTION
Choice of Solution
| Solution | Abbreviation |
|---|---|
| Lactated Ringer’s | LR |
| Sterile water for injection | SW |
| 5% dextrose in water | D5W |
| Sodium chloride injection |  NS NS |
Although other infusion solutions are available, these represent the most commonly used solutions.
The answer is that a 5% dextrose and water solution is not contraindicated in the diabetic patient. First, the concentration of dextrose (5%) is not great enough to produce any significant change in the blood sugar level of this patient.1 Second, as stressed in Chapter 26, the patient receiving IV moderate sedation will be requested to fast (be NPO) for approximately 4 hours before the planned procedure. The patient arrives at the dental office with a decreased blood sugar level, perhaps not quite hypoglycemic but definitely not hyperglycemic. The addition of approximately 250 to 500 ml of 5% dextrose and water will produce a slight elevation in blood sugar level, a desirable effect at this time. It must be remembered that when a person with diabetes becomes clinically hypoglycemic, treatment of choice is the administration of 50% dextrose, a solution 10 times that which is infused during IV sedation.
Volume of Solution
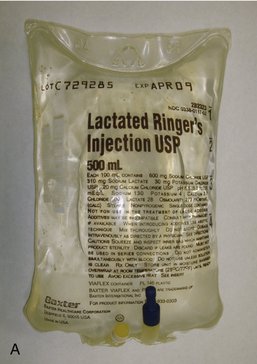
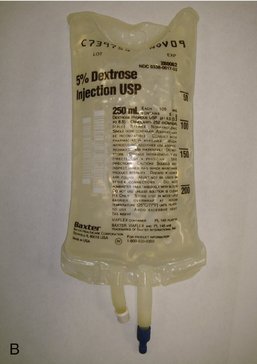
All IV infusion solutions (fluids) are packaged in plastic bags (Figure 22-4).
The 250-ml or 500-ml bags of IV fluid represent the most nearly ideal sizes for the typical 1- to 4-hour dental IV moderate sedation procedure. With proper management of the flow rate (as discussed in Chapter 26), a 250-ml or 500-ml bag can be made to last for 3 to 4 hours.
The solution found within the IV bag is sterile. However, problems with contaminated fluids have developed in the past.2 Care must be exercised by the user of such fluids to try to ensure their continued sterility. Administration of contaminated fluids directly into the cardiovascular system of the patient can produce bacteremia or septicemia and has led to deaths and significant morbidity.3
The following should be checked before using a bag of solution:
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses