Dental Implants
History of Dental Implants
The recognition of failure rates of transplants increased interest in the implantation of artificial tooth roots. In 1809 Maggiolo fabricated gold roots that were fixed to teeth by means of a spring. These gold implants were placed into fresh extraction sites, although they were not truly submerged into bone. The crowns were placed after healing had occurred around the implant. Many attempts followed. Harris (1887) proposed the use of a platinum post coated with lead. The post was shaped like a tooth root, and the lead was roughened for retention in the socket. Bonwell (1895) implanted gold or iridium tubes to restore a single tooth or support complete dentures. Payne (1898) implanted a silver capsule as a foundation for a porcelain crown that was cemented after weeks. Scholl (1905) demonstrated a porcelain corrugated root-shaped implant that lasted for two years and was anchored to adjacent teeth by pins. Greenfield (1913) introduced and patented a hollow “basket” implant made of a meshwork of 24-gauge iridium-platinum wires soldered with 24-karat gold. This device was used to support single implants as well as fixed dental prostheses comprising as many as eight implants.
Consistent problems with these artificial implant designs and materials supported the need for a scientific approach to implant selection and placement. Some have proposed that the “modern era” started in 1925. In 1937 Venable et al. analyzed the interactions of cobalt alloy and other available metals and alloys with bone. They concluded that certain metals produced a galvanic reaction, which led to corrosion when these metals contacted tissue fluids. They proposed the use of Vitallium, a cast alloy, which was composed of cobalt, chromium, and molybdenum. This alloy was considered to be relatively inert, compatible with living tissue, and resistant to the adverse reactions with body fluids. Vitallium has also been used in different forms of surgical devices, such as dental subperiosteal implant and orthopedic plates, screws, nails, and joints. Early evaluations documented Vitallium implants with survival times of 10 or more years.
Classification of Implants
Implant Design
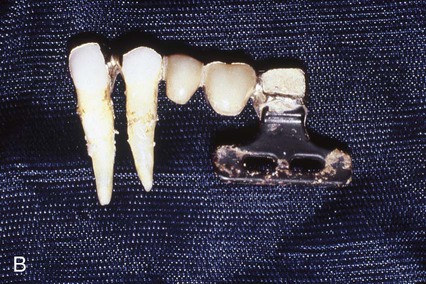
The first and most commonly used type of design is the endosteal (called endosseous) implant, a device placed into an alveolar and/or basal bone of the mandible or maxilla that usually transected only one cortical plate. These implants were formed in many different shapes, such as root-form cylindrical cones or screws or thin plates called plate or blade forms, and they were used in all areas of the mouth. One example of an endosteal implant was called the blade implant (Figure 20-1), which was developed independently in 1967 by two groups led initially by Roberts (1970) and Linkow (1968). Endosteal blade implants consisted of thin plates placed into bone; they were most commonly used for narrow anatomic structures such as posterior edentulous areas after significant resorption of bone. Because of various issues with blade implants, their application in more recent implantology has decreased. Another example of an endosteal implant was the ramus frame implant, a horseshoe-shaped stainless steel device inserted into the ascending rami (bilateral) and the anterior symphysis area of the mandible. As with the blade implants, the number of applications have been limited. The most popular endosteal implant has been the root-form (Figure 20-2), which was designed initially to mimic the shape of tooth roots for directional load distribution as well as for positioning in bone. In longitudinal studies, the root-form implant has the most documentation of the endosteal implants, although several surgical stages may be needed for completion.
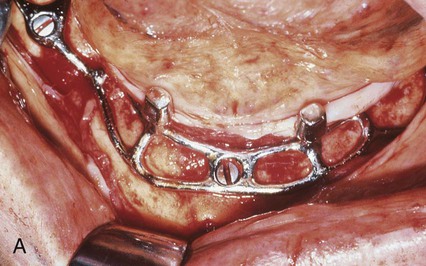
The second implant design was the subperiosteal implant (Figure 20-3), which employed an implant substructure and superstructure. The custom-cast frame was placed directly beneath the periosteum overlying and fitting along the bony cortex. This implant was first developed by Dahl (1943) and refined by Berman (1950), who used a direct bone impression technique. These devices were used to restore partially dentate or completely edentulous jaws when there was inadequate bone for endosseous implants. Use of the subperiosteal implant has been limited because of numerous considerations, including the difficulty of retrieval.
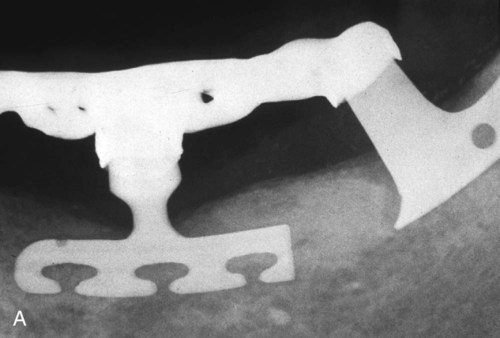
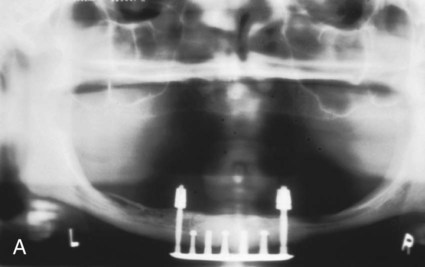
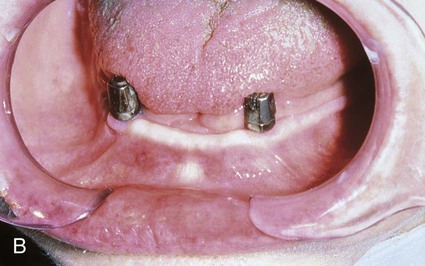
The third design was the transosteal implant (Figure 20-4), which combined subperiosteal and endosteal components. This type of implant penetrated both cortical plates and passes through the full thickness of the alveolar bone. Use of the transosteal implant has been restricted to the anterior area of the mandible and provides support for tissue-borne overdentures. The concept of transosseous implants was first conceived in Germany in the early 1930s; early examples were made of a cobalt alloy. Small (1968) developed the mandibular staple implant made of a titanium alloy, which was modified by Bosker (1983), who produced the transmandibular implant (TMI) made of a gold alloy. Other names for transosteal implants have included staple bone implant, mandibular staple implant, and transmandibular implant.
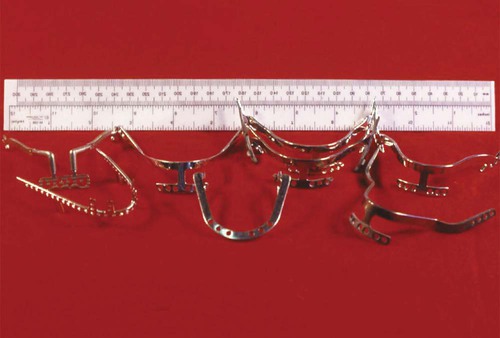
From a historical and applications perspective, these systems were reviewed in the early 1970s by Natiella et al. (1972) and subsequently in each decade by researchers who participated in professional society–based consensus conferences. To summarize the various biomaterials and designs tested for dental implants, examples of devices received for examination prior to 1990 are shown in Figures 20-5 through 20-7.
Implant Properties
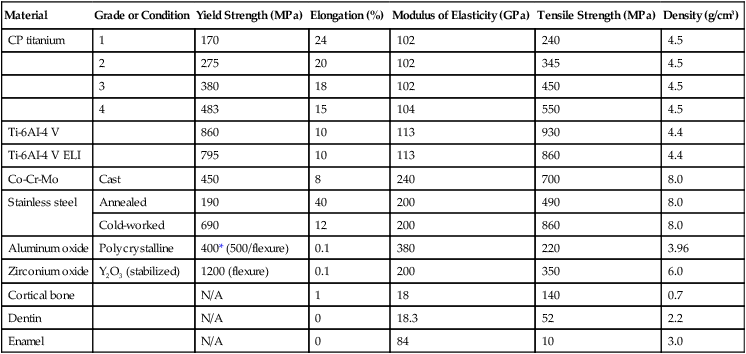
Implant biomaterials can also be classified according to their composition and their physical, mechanical, chemical, electrical, and biological properties. These classifications often include ranked comparisons of properties such as elastic moduli, tensile strength, and ductility to determine optimal clinical applications (Table 20-1). These properties are used to aid in the design and fabrication of the prosthesis. For example, the elastic modulus of the implant is inversely related to the strain transferred across the implant-tissue interface during loading of the implant; that is, the greater the elastic modulus of an implant, the greater is the stress in the implant and the lower is the stress transferred to bone.
TABLE 20-1
Mechanical Properties and Density of Metallic and Ceramic Implant Materials
| Material | Grade or Condition | Yield Strength (MPa) | Elongation (%) | Modulus of Elasticity (GPa) | Tensile Strength (MPa) | Density (g/cm3) |
| CP titanium | 1 | 170 | 24 | 102 | 240 | 4.5 |
| 2 | 275 | 20 | 102 | 345 | 4.5 | |
| 3 | 380 | 18 | 102 | 450 | 4.5 | |
| 4 | 483 | 15 | 104 | 550 | 4.5 | |
| Ti-6AI-4 V | 860 | 10 | 113 | 930 | 4.4 | |
| Ti-6AI-4 V ELI | 795 | 10 | 113 | 860 | 4.4 | |
| Co-Cr-Mo | Cast | 450 | 8 | 240 | 700 | 8.0 |
| Stainless steel | Annealed | 190 | 40 | 200 | 490 | 8.0 |
| Cold-worked | 690 | 12 | 200 | 860 | 8.0 | |
| Aluminum oxide | Polycrystalline | 400* (500/flexure) | 0.1 | 380 | 220 | 3.96 |
| Zirconium oxide | Y2O3 (stabilized) | 1200 (flexure) | 0.1 | 200 | 350 | 6.0 |
| Cortical bone | N/A | 1 | 18 | 140 | 0.7 | |
| Dentin | N/A | 0 | 18.3 | 52 | 2.2 | |
| Enamel | N/A | 0 | 84 | 10 | 3.0 |
Tissue Integration
Implant-supported restorations differ from tooth-supported restorations in that the former lack a periodontal ligament, which reduces shear stress and strain, provides shock absorption, and reduces the development of dangerously high occlusal forces. These benefits of the periodontal ligament reduce the potential for inadvertent damage to the tooth or restoration. With the increased popularity of all-ceramic restorations, the effect of no periodontal ligament attachment in implant-supported restorations must be established. Ceramic materials are brittle by nature and because of inherent processing flaws, they cannot withstand any stress above their yield point, which is equivalent to their tensile strength. These stresses can be generated during occlusal loading. Implants, on the other hand, are directly anchored to the bone and cannot readily flex laterally under extremely high occlusal loads to relieve some of the excessive loading. Based on an in vitro study, Vult von Steyern et al. (2005) analyzed the fracture strength of all-ceramic prostheses on abutment teeth and implants. They concluded that implant-supported ceramic prostheses fractured at higher loads than those supported by natural teeth because of the lack of a periodontal ligament. To date, there are no clinical studies comparing the performance of ceramic FDPs supported by implants and natural teeth.
Implant Components
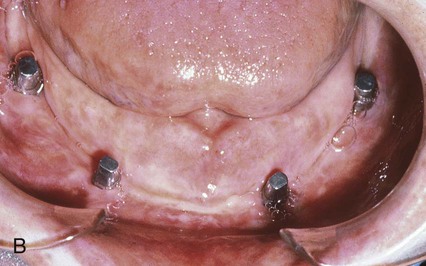
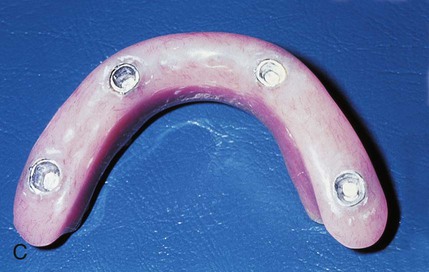
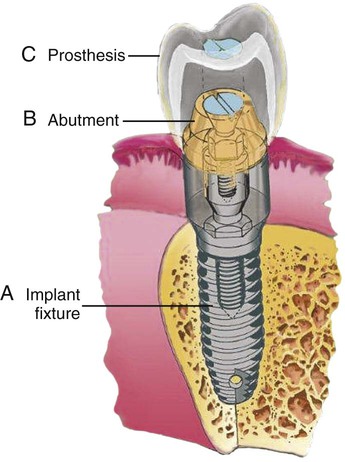
To understand the material characteristics and function of an implant, one must first be knowledgeable about its numerous component parts. Although each implant system varies, the parts are basically consistent. The body of the implant (called a fixture for the Brånemark system) (Figure 20-8, A) is the implant component that engages with bone. Depending on the implant system, the body section can have different surfaces—threading, grooved, perforated, plasma-sprayed, or coated. These characteristics are often classified as subtraction (acid etch) or addition (coating) types. Each surface type is meant to serve a particular purpose—for example, increased surface area enhances bone integration, and better cortex engagement plays an important role in immediate and long-term bone anchorage. The coated or plasma-sprayed biomaterials, discussed later in this chapter, are used to enhance attachment to bone. The second component (Figure 20-8, B) is the transmucosal abutment, which provides the connection between the implant body and the intraoral prosthesis to be fabricated (Figure 20-8, C), which will provide intraoral function. The abutment is usually connected to the implant body by means of a screw; however, it can also be cemented or connected by a Morse taper-type design. Abutments can become engaged to the implant body either by an internal or external geometry (initially a hexagon) within the implant body, which also serves as an antirotation device and is particularly important for single-unit restorations. The last part of an implant is the prosthesis. This can be attached to the abutments through the use of screws, cement, precision attachments, magnets, or other designs, such as those used for removable implant overdentures.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses