Chapter 2
Top 60 most important medications used in an orofacial pain treatment center
2.1 What Is Chronic Orofacial Pain? What Is Pharmacologic Treatment Success?
As described in Chapter 1, there are many orofacial pain (OFP) diseases, disorders, and dysfunctions and several review articles have described them.1–8 The purpose of this chapter is to introduce and briefly review the 60 top pharmacologic treatments provided for patients with chronic OFP. While there certainly are many more than 60 medications used to help manage painful orofacial conditions, we have elected to focus on the top 60. Before reviewing the relative efficacy and evidentiary basis of these 60 medications it is appropriate to explain that the majority of patients with chronic OFP will not find a “cure” to their pain with medications but might, with medications added to physical and behavioral treatment methods, find a way to manage their pain. Some patients will ask the question, “How long will I have to take these medications?” If they were being treated for diabetes or hypertension, this question would be not be logical because these two diseases, like chronic pain, are not usually cured, but instead are managed with medications. A 2005 study examined what defines treatment success from the patient’s perspective.9 Specifically this study asked chronic-pain patients (n = 110) what they would consider a success on four dimensions (pain, fatigue, emotional distress, interference with daily activities). They described that the mean level of pain, fatigue, emotional distress, and interference with daily activities was moderately high at their first visit to the clinic, and these patients reported they would consider their treatment “successful” if their pain scores were reduced between one-half and two-thirds. The problem is that, although patients and doctors expect and hope for this level of change, the actual long-term results from treatment of chronic OFP are more modest in a large percentage of patients. The general rule with chronic pain is that the longer a patient has the pain, the lower the reduction in pain achieved with treatment.
This point is illustrated by two studies on the long-term outcomes of patients in an OFP treatment center. The first study reported on 109 consecutive patients seen in a chronic-OFP clinic.10 This group of patients had between 4 and 9 years from their first visit to the follow-up; of the 109, 85% responded to the questionnaire. The bad news was that only 27% of patients experienced total disappearance of pain and the remaining 73% still had ongoing pain. A second study examined the outcome of a cohort of 74 patients suffering chronic idiopathic facial pain who were first seen at a chronic pain center a minimum of 9–19 years prior.11 Of the 74 cases eligible for follow-up, 13 patients had died and 16 did not wish to participate; of the 45 remaining patients, 10 out of 45 (22%) reported that they were free of orofacial pain at follow-up and, similar to the prior study, the remaining 78% reporting ongoing pain. Based on these two studies the best that can be said is that a full cessation or cure of chronic OFP with treatment is between 22% and 25%. It almost goes without saying that the relative mix of diseases in the OFP-clinic population, the methods of treatment and the medications used, and, most important, the ability of the clinicians to explain and render care would greatly influence the long-term results. The message taken from these two studies is that most patients with chronic orofacial pain are managed not cured.
2.2 What Are the Top 60 Medications Used to Manage Chronic Orofacial Pain?
The 60 medications included in this chapter were selected because they are commonly utilized “pain” medications, but it is also clear that the evidentiary basis for using these medications to treat orofacial pain is limited. To illustrate this point, we searched Medline, cross-referencing the name of the drug with the words (1) pain, (2) facial pain, and (3) orofacial pain. The results (Table 2.1) show that there are many studies linking these drugs to the pain literature, but there are relatively few literature citations where these medications have been linked with OFP disorders. Another example of this point is a study published in 1995 that examined the literature available for treatment of temporomandibular disorders (TMDs).12 This meta-analysis examined the literature from 1980 to 1992 and found more than 4000 references; however, among these only 15% were clinical studies, and only approximately 1% (N = 55) were randomized controlled trials that provided the type of evidence usually considered essential for evaluating the efficacy of a therapeutic modality. Based on this, the authors concluded that it was not clear whether any of the therapies currently in use for TMDs provided any benefit over placebo alone.
Table 2.1 Time-delimited Medline search (10 years, January 1, 1997–December 31, 2007)
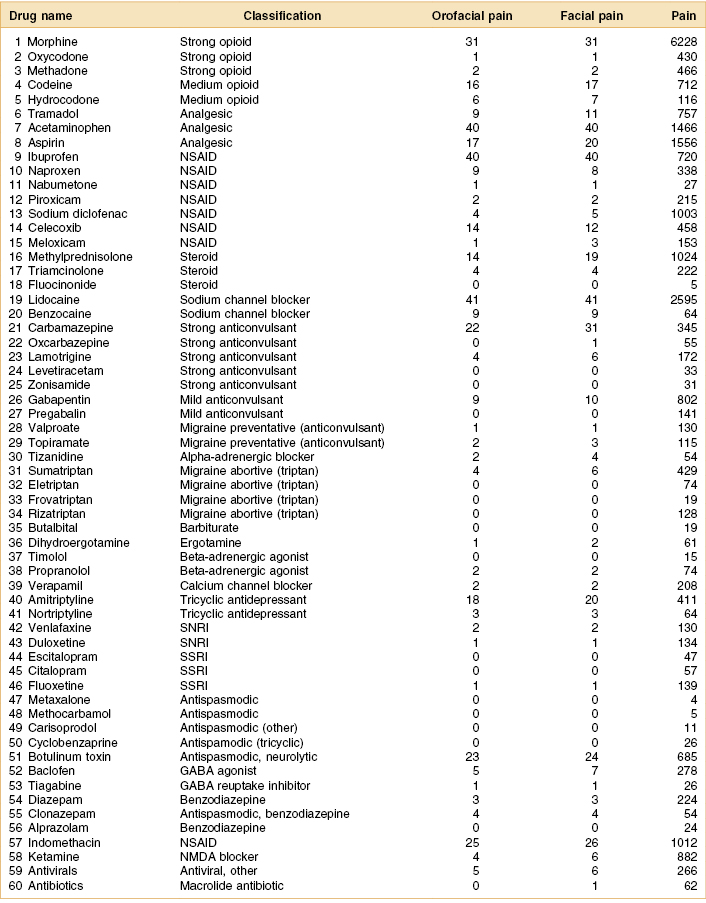
GABA, gamma-aminobutyric acid; NMDA, N-methyl-D-aspartate; NSAID, nonsteroidal anti-inflammatory drug; SNRI, serotonin–noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
2.3 What Has the Recent Literature Said About Pharmacologic Treatment of Chronic Orofacial Pain?
The issue of what medications are useful for TMD–OFP and various other orofacial pain disorders has been addressed in two review articles. First, a 1997 paper focused on pharmacologic therapy for TMDs and reviewed nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, antidepressants, muscle relaxants, hypnotics, and anxiolytics.13 The review found little data on the long-term use of NSAIDs and quite a few reports on the potential side effects of these medications used in this fashion. It suggested that a short trial of an NSAID may be considered in patients with an apparent inflammatory component to their pain complaint but that after 2 weeks, if great benefit is not achieved, they should be discontinued. Regarding the use of opioids for pain, this review suggested that further studies are needed but this class of drugs has potential for those patients with chronic severe OFP. Of course, careful patient selection is necessary to rule out drug-seeking behavior or other personality disorders; careful monitoring is needed to individualize dose, thereby minimizing side effects and dose escalation; and careful attention must be paid to regulatory procedures. Regarding the use of antidepressants for chronic nonmalignant OFP, the review concluded that tricyclic antidepressants (e.g., amitriptyline or doxepin) were potentially effective used in the lower dose range. The dose of antidepressants will usually be limited by anticholinergic side effects (dry mouth, constipation, blurred vision, and urinary retention) and should be adjusted in response to individual variation in analgesic response and side effects. Regarding the use of benzodiazepines, the review was neither supportive of nor opposed to their value in treating chronic OFP, and it suggested they should not be prescribed in large amounts and that careful monitoring for dose escalation and undue dependence on these medications was warranted. This review suggested they not be used in patients with depression. However, for cases of muscle pain and trismus, they can be used but only for a 2- to 4-week course. Regarding more traditional skeletal muscle relaxants for OFP-based myogenous pain and trismus, the review concluded that these medications, like the benzodiazepines, are best used only for a brief time (e.g., 2 weeks) and in conjunction with physical therapy regimens.
In 2003, another systematic review of the literature was published that assessed the pain-relieving effect and safety of pharmacologic interventions in the treatment of chronic TMDs, including rheumatoid arthritis (RA), atypical facial pain (AFP), and burning mouth syndrome (BMS).14 The study reported on randomized clinical trials (RCTs) on adult patients with these diseases. They found a total of 11 studies—with a total of 368 patients who met the inclusion criteria—and concluded that amitriptyline was effective in 1 study and benzodiazepine in 2 studies. They described one study that showed intra-articular injection with glucocorticoid relieved the pain of RA of the temporomandibular joint (TMJ) and another that showed the combination of paracetamol, codeine, and doxylamine was effective in reducing chronic TMD pain. Finally, this review found no effective pharmacologic treatment for BMS and interestingly only minor adverse effects were reported in these studies. The conclusion, drawn from these two review articles, is that most chronic pain medications, other than opioids, do not provide a strong therapeutic benefit and it is also critical to assess the balance between therapeutic benefit and safety for each drug for each patient.
2.4 Why Should We Be Cautious About the Current Literature?
As can be seen in Table 2.1, there is a great paucity of studies on medications used specifically for orofacial pain management. Among those that exist, many are methodologically flawed and the population of patients with OFP studied was very heterogeneous. Patients with myogenous pain, for example, are often not distinguished in clinical trials from those who have TMJ disorders such as degenerative arthritis or displacement of the meniscus.15,16 Observations by clinicians and case series often fail to use standardized methods for measurement of pain and dysfunction. The main evidence of a positive treatment outcome is too often the clinician’s impression of improvement or the patients’ failure to seek further treatment.17,18 Another major weakness in previous studies has been the lack of an adequate control group receiving either a placebo, a drug with known efficacy as a positive control, or no treatment. These deficiencies in study design are particularly significant given the high rate of success reported for manipulations such as placebo splints, placebo drug, sham occlusal equilibration, a positive doctor–patient relationship, and enthusiastically presented treatment.19–21 Another factor that may affect the evaluation of treatment outcome in response to drug therapy is the fluctuating nature of orofacial pain, which may undergo remissions and exacerbations independent of treatment. The high incidence of concurrent psychological problems described in this population may also influence the onset of symptoms, reporting of pain levels, and treatment response.22–24 For some disorders, especially those that are not neuropathic in character, many patients eventually improve even if an initial course of therapy is not successful or if they receive no treatment at all.25 The pharmacologic management of OFP rests on the same principles that apply to all other drugs: demonstrated efficacy for the indication (chronic OFP), an acceptable side-effect liability, and safety when given for prolonged periods.
Now, if you stopped reading at this point you might conclude that few medications are proven and even fewer should be used for chronic OFP. However, this is not the case and a quick visit to a chronic pain or headache center shows that they use multiple medications to help their patients. These medications are usually given in a series of titration trials to see if the patient achieves substantial benefit without remarkable side effects. When this happens, patients’ lives are changed for the better. Therefore, this chapter provides a partial description of the characteristics and possible use of the top 60 pain-related medications and reviews some of the current evidence supporting their use for the chronic OFP disorders. Detailed information about each of the 60 drugs reviewed here is provided in subsequent chapters.
2.5 Drugs 1–5: Opioids (Morphine, Oxycodone, Methadone, Codeine, Hydrocodone)
The first and most important category of medications for chronic pain relief is the natural and synthetic derivatives of the opium plant, labeled opioids. These medications provide pain relief because they bind to opiate receptors in the central nervous system (CNS), thus altering pain perception. Unfortunately, the opiate receptors produce other effects leading to physical and emotional dependence on these drugs with prolonged use. Among the five opioids listed here, the most commonly used in an outpatient OFP clinic are hydrocodone and codeine drugs. In the United States, hydrocodone and codeine manditorily come in combination with nonopioid analgesics when prescribed. The most common combination is with acetaminophen, aspirin, or ibuprofen. The stronger opioids (morphine, oxycodone, and methadone) are prescribed as stand-alone analgesic agents, although oxycodone also can be prescribed combined with nonopioid analgesics. There are certainly some patients attending an OFP center who are candidates for morphine, oxycodone, or methadone, especially those patients with neuropathic pain that cannot be controlled with nonopioid analgesics, anticonvulsants, and other adjunctive pain analgesics. While opioids are powerful and have a proven efficacy at reducing pain, the long-term consequence of using opioids for nonmalignant pain is controversial. One recent study examined the long-term effects of opioids on pain relief, quality of life, and functional capacity in long-term or chronic noncancer pain and reported that, while pain is certainly managed with these agents, these patients are not cured and still have substantial problems plus the additional problem caused by using a drug that produces a powerful physical dependence.26 For these reasons, the chronic use of opioids for patients with persistent orofacial pain requires careful patient selection to rule out those patients who might be exhibiting drug-seeking behavior or other personality disorders that would make opioid contraindicated. Logically any patient who is a candidate for opioid use must fully understand the drug-dependence issues that long-term use entails. When opioids are used, the cautious clinician will perform careful periodic monitoring of the patient while individualizing the patient’s dose. Steps that a pain-knowledgeable dentist or physician should follow when prescribing opioid medications are given in Chapter 4. Only by this process can side effects be minimized, and abuse and dose escalation prevented.
2.6 Drug 6: Analgesic (Tramadol)
Tramadol is a centrally acting synthetic codeine analog that was approved by the US Food and Drug Administration (FDA) in 1995 for moderate to moderately severe pain. It is not categorized as a Schedule II or III drug and is currently categorized as a nonopioid analgesic, so it does not have a narcotic schedule classification. For all of these reasons, tramadol is being discussed seperately from the other opioids. Tramadol comes either alone or in combination with nonopioid analgesics such as aspirin, acetaminophen, and ibuprofen. Even though it is classified by the FDA as a nonopioid analgesic, this drug does bind to the μ-opioid receptor in the CNS. It also acts like a tricyclic antidepressant agent causing inhibition of serotonin and norepinephrine at the synaptic cleft.27,28 The effects of these actions (μ-opioid binding and serotonin–norepinephrine reuptake inhibition) both produce inhibition of the ascending pain signals and can activate the descending pain inhibitory pathway. Tramadol’s opioid affinity and activity are also substantially less than those of morphine. Due to tramadol’s (albeit weak) opioid activity, there have been questions about potential abuse. A proactive surveillance program revealed that the vast preponderance of patients who abuse tramadol have a previous record of substance abuse.29
2.7 Drugs 7 and 8: Analgesics (Acetaminophen, Aspirin)
The World Health Organization (WHO) recommends nonopioid analgesics for the initial treatment of pain. The three most common analgesics that do not have opioid receptor binding action are aspirin, acetaminophen, and the nonsteroidal anti-inflammatory drugs (NSAIDs). Generally the WHO analgesic ladder is designed for acute pain management and unfortunately this organization does not modify its recommendations for chronic pain. This is a problem since, although aspirin (acetylsalicylic acid) is an important analgesic for acute pain, it does not appear appropriate for chronic pain use because of the known gastropathic-inducing side effects (gastric irritation and nausea). The same concern (induced gastropathic disease) also exists for NSAIDs. Nevertheless, aspirin is widely available and used for pain since it is an over-the-counter product. The primary mechanism of action of aspirin is that it inhibits prostaglandin synthesis and acts on the hypothalamus to reduce fever. When nociceptive fibers are being stimulated by an endogenous inflammatory reaction in the peripheral injury site, prostaglandin is a critical component of the inflammatory cascade of events. For this reason inflammatory pain is effectively blocked by aspirin. A review article on aspirin as a postoperative analgesic suggests it is effective but has substantial side effects, even in short-term use.30 This meta-analysis examined 72 studies where aspirin was compared with other analgesic agents or placebo agents. These studies included in total over 6550 subjects divided between those receiving placebo and those getting the active agents. These studies were all short term because the primary use of aspirin is for postoperative pain. Aspirin was found to be significantly superior to placebo with single oral doses of 600 or 650 mg, 1000 mg, and 1200 mg.
Of course aspirin is used by patients with chronic pain and especially by patients with episodic pain due to headache, sometimes resulting in benefit and sometimes harm. One study examined the efficacy and tolerability of aspirin versus placebo for the acute treatment of a single acute attack of migraine.31 This prospective, randomized, double-blind, parallel-group, placebo-controlled study evaluated the efficacy of a single, 1000-mg dose of aspirin for the treatment of acute moderate-to-severe migraine, with or without aura. Again this study examined only the short-term efficacy of aspirin, looking at headache pain response at 2 hours. Of 485 enrolled subjects with migraine attacks, 201 used aspirin and 200 used placebo. The 2-hour headache response rate was 52% with aspirin versus 34% with placebo (P < 0.001). Aspirin was significantly more effective than placebo for pain reduction beginning 1 hour after dosing (P < 0.001) and continuing throughout the 6-hour evaluation period. This study demonstrated that aspirin used in this fashion was safe and effective for treatment of acute migraine in appropriately selected patients.
Acetaminophen is another over-the-counter nonopioid analgesic used by pain patients. Like aspirin, this drug is an important analgesic for acute pain and if used at levels that are nontoxic, it can be used for chronic pain. Although acetaminophen does not cause gastropathy as a side effect, the major concern is that it is not uncommon for patients to inadvertently take more than the maximum daily dose (4000 mg/day) and produce a liver toxicity that causes rapid irreversible liver damage, which can be fatal.32 Acetaminophen’s primary mechanism of action is that it inhibits prostaglandin in the CNS and peripherally blocks pain-impulse generation, and it acts on the hypothalamus to reduce fever.33 A recent meta-analysis examined this drug, assessing 46 clinical studies that compared acetaminophen and placebo.34 These studies in total included 2530 subjects who received acetaminophen and 1594 who received placebo, and its value above and beyond placebo is well established. Both aspirin and, to a much greater extent, acetaminophen and its European equivalent, paracetamol, are used as headache abortive agents; depending on the frequency of the headaches, this can mean daily use of these drugs. A recent study examined the effectiveness of a nonprescription combination of acetaminophen, aspirin, and caffeine at alleviating migraine headache pain.35 The study was a triple double-blind, randomized, parallel-group, single-dose, placebo-controlled experiment that included migraineurs with moderate or severe headache pain. The study enrolled 1357 patients; 1250 took study medication and 1220 were included in the efficacy-evaluable data set. The results showed that significantly greater reductions in migraine headache pain intensity occurred 1–6 hours after dose in patients taking the acetaminophen–aspirin–caffeine combination than in those taking placebo. Pain intensity was reduced to mild or none 2 hours after dose in 59.3% of the 602 drug-treated patients compared with 32.8% of the 618 placebo-treated patients (P < 0.001). In addition to the obvious efficacy, this drug combination also has an excellent safety profile and is well tolerated. Unfortunately, because it has a good effect for episodic headaches, over-the-counter analgesic medication sometimes is overused and this can lead to a disorder called medication overuse headache. The basic concept behind this is that analgesic use can cause central sensitization of the trigeminal and somatic nociceptive systems, and these changes are thought to occur in the cerebral supraspinal structures.36
2.8 Drugs 9–15: NSAIDs (Ibuprofen, Naproxen, Nabumetone, Piroxicam, Sodium Diclofenac, Celecoxib, Meloxicam)
In this category, we have selected for inclusion five commonly used nonspecific cyclooxygenase (COX) inhibiting nonsteroidal anti-inflammatory drugs for arthritis pain (ibuprofen, naproxen, nabumetone, piroxicam, and sodium diclofenac) and two cyclooxygenase-2 (COX-2) specific inhibiting medications (celecoxib, meloxicam). Like aspirin, these drugs are used for acute pain and for phasic arthritic pain. The primary mechanism of action of all of the NSAIDs reviewed here is that they inhibit prostaglandin synthesis by decreasing the activity of the cyclooxygenase enzyme. The main drawback of the five nonspecific COX-inhibiting NSAIDs when used continuously is that they cause gastropathy (gastric irritation and nausea).37 Retrospective studies have established an association between increased risk of upper gastrointestinal bleeding and ingestion of aspirin or NSAIDs.38–40 This side effect is less likely with the two COX-2 inhibitors, but they have the added side effect of an increased risk of cardiac damage.41 Nevertheless, NSAIDs are used widely for both headache and arthritic pain since two of them (ibuprofen and naproxen) are available as an over-the-counter product. Considering the adverse effects of long-term use of NSAIDs, and the lack of clinical evidence demonstrating a therapeutic effect for these nonopioid analgesics in the symptomatic treatment of myalgia or fibromyalgia, this must be weighed against the potential for serious toxicity with chronic use for myogenous-based disease.
A short trial of an NSAID may be considered in patients with an apparent TMJ inflammatory component to their pain complaint, but a lack of therapeutic effect after a 7- to 10-day trial or the development of any gastrointestinal symptoms should prompt discontinuation of the NSAID. Patients with risk factors for gastrointestinal or kidney disease should be managed cautiously with NSAIDs or acetaminophen and should not take these drugs for prolonged periods of time. For those patients with gastritis the possibility exists for them to use a topical NSAID, and a recent study examined the efficacy and tolerability of a topical ketoprofen patch in the treatment of uncomplicated ankle sprain.42 Of course it would be more relevant if such data were available for TMJ strain, but such data is not available. Nevertheless, for ankle strain, a randomized, double-blind, placebo-controlled, multicenter, 2-week trial was performed on 163 subjects. Pain levels were the primary outcome measure and it was found that the ketoprofen patch was better than placebo. Specifically ketoprofen demonstrated a greater reduction in pain after 7 days than those assigned to placebo. Adverse events (mostly local skin reactions) occurred in 30.9% of the ketoprofen group and in 24.4% of the placebo group.
The safety of COX-2 selective NSAIDs such as celecoxib and meloxicam has received great attention in recent years. A 2008 review examined the clinical effectiveness of several COX-2 selective NSAIDs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib, and lumiracoxib) for osteoarthritis (OA) and rheumatoid arthritis (RA).43 This review included only randomized controlled trials and the authors concluded that, although the COX-2 selective NSAIDs as a class of medications offered protection against serious gastrointestinal events, the amount of evidence for this protective effect varied considerably across individual drugs. The relative cardiovascular safety also varied substantially between COX-2 selective NSAIDs. An increased risk of myocardial infarction (MI) compared with nonselective NSAIDs was observed among those drugs with greater volume of evidence in terms of exposure in patient-years. There is no study that has examined meloxicam for TMJ-related arthritis or pain, but a 2004 study on TMDs did examine the relative efficacy of celecoxib versus naproxen and placebo in a randomized controlled clinical trial.44 This study included 68 subjects with painful TMJs secondary to disk displacement with reduction (DDWR). The results showed that naproxen significantly reduced the symptoms of painful TMJ–DDWR as determined by most efficacy measures and also showed a significant improvement in pain intensity during the study. Celecoxib and naproxen were equally well tolerated, with similar numbers of reported adverse effects. In conclusion, the final choice to use a COX-2 selective NSAID or a nonselective NSAID is left up to the practitioner, who will weigh the risk versus benefit of the medication.
2.9 Drugs 16–18: Corticosteroids (Methylprednisolone, Triamcinolone, Fluocinonide)
Three commonly used corticosteroids are methylprednisolone, triamcinolone, and fluocinonide. The first agent is often given systemically or via injection for acute pain and inflammation.45 The second agent is also available for systemic use, but it is more commonly used as an intracapsular injection for joint pain or as a topical application for skin reactions where inflammation is present. These agents are powerful anti-inflammatory agents and, like aspirin, are used for acute pain and even sometimes for chronic pain, but they are not specifically FDA approved for pain. They are approved for a wide variety of inflammatory diseases, including autoimmune disease (e.g., erosive lichen planus, pemphigus, graft-versus-host disease, rheumatoid arthritis). Like aspirin and NSAIDs these agents when used continuously will cause gastropathy (gastric irritation and nausea) as well as many other major side effects. Both methylprednisolone and triamcinolone are generally used short term either as a systemic dose for inflammatory disease or as an injectable agent for arthritic pain. Only occasionally will these agents be used chronically and then in generally lower doses. The primary mechanism of action of these two agents is to decrease inflammation by suppression of migration of leukocytes and reversal of increased capillary permeability. By producing a general suppression of the immune system, inflammatory-related pain is effectively blocked.
The third corticosteroid in this category is fluocinonide, and a recent double-blind clinical trial examined the efficacy of topical steroids for treatment of chronic oral vesiculoerosive disease.46 This study compared two potent topical corticosteroids (clobetasol propionate and fluocinonide ointment in orabase) as treatments for controlling oral vesiculoerosive diseases. Sixty patients were included (43 women and 17 men) and final data were available for 55. The study duration was 28 days and outcomes included pain, erythema, atrophy, and size of lesion. The results showed that both medications had a beneficial effect in the control of symptoms and signs of oral vesiculoerosive diseases with minimal side effects, although candidiasis was observed in 13 patients at the end of treatment in this population. The authors suggested concurrent antifungal therapy is indicated in some cases.
2.10 Drugs 19 and 20: Local Anesthetics and Sodium Channel Blockers (Lidocaine, Benzocaine)
The anesthetics lidocaine and benzocaine are both membrane stabilizing agents that work by blocking voltage-gated sodium channels. Local anesthetic agents have been shown to effectively treat neuropathic pain in animal models.47 Clinically, neuropathic pain states respond transiently to intravenous infusion of lidocaine, but unfortunately the effect is only present during the infusion. There are two clinically available cutaneous local anesthetic preparations: (1) EMLA cream (AstraZeneca, Wayne, PA), which is a eutectic mixture of the local anesthetics lidocaine and prilocaine, and (2) Lidoderm (Endo Labs, Chadds Ford, PA), which is a 5% lidocaine patch.48,49 Although EMLA is useful for venipuncture and cutaneous biopsy, it has not found a role in chronic pain management.50 In contrast, the topical 5% lidocaine patch may be useful in management of peripheral neuropathic pain conditions. An open-label trial showed that the patch gave moderate or better pain relief in 81% of a small group of patients with cutaneous refractory neuropathic pain states.51,52 Controlled studies are continuing, but the Lidoderm patch has been approved by the FDA for treatment of postherpetic neuralgia. The dose is one patch to the affected area every 12 hours, and serum levels are insignificant. In general lidocaine and even benzocaine are safe to use topically, but there is a risk of methemoglobinemia.53
2.11 Drugs 21–25: Anticonvulsants (Carbamazepine, Oxcarbazepine, Lamotrigine, Levetiracetam, Zonisamide)
In this category are five antiepileptic drugs (AEDs), which are also called anticonvulsants, that are known to depress abnormal neuronal discharges and raise the threshold for propagation of neural impulses. AEDs have been found to have therapeutic efficacy in all neuropathic pain, including orofacial neuropathic pain states. The most frequently used is carbamazepine, which has been the drug of choice, for many years, for treating trigeminal neuralgia.54 These agents do not have an FDA narcotic schedule classification but have significant clinical toxicity nonetheless. These five agents reviewed here (carbamazepine, oxcarbazepine, lamotrigine, levetiracetam, zonisamide) are approved for control of epileptic seizures, and carbamazepine is approved for trigeminal neuralgia as well. Carbamazepine and oxcarbazepine are the mainstay of trigeminal neuralgia therapy. Oxcarbazep/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


