Chapter 2
The dentin–pulp complex: structures, functions and responses to adverse influences
Introduction
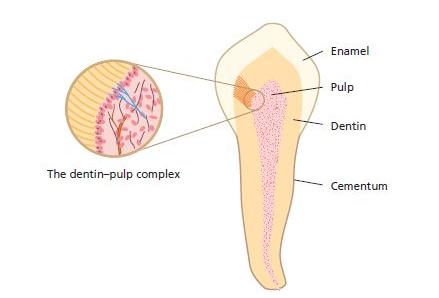
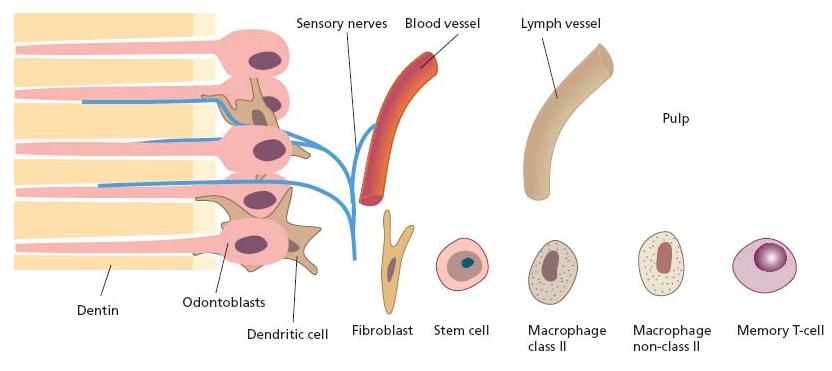
The extent to which the dental pulp will sustain impairment in the clinical setting depends on its potential to oppose bacterial challenges and withstand injury by various forms of trauma. To understand the biological events that operate and most often prevent the pulp from suffering a permanent breakdown, the specific biological functions of both dentin and pulp under pathophysiological conditions will be addressed in this chapter. These two tissue components of the tooth form a functional unit that often is referred to as the dentin–pulp complex (Fig. 2.1).
Constituents and normal functions of the dentin–pulp complex
Dentin and dentinal tubules
Dentin provides elasticity and strength to the tooth that enable it to withstand loading forces by mastication and trauma. Dentin also elicits important defense functions aimed at preserving the integrity of the pulp tissue.
Under normal, healthy conditions, when dentin is covered by enamel and cementum, fluid in the dentinal tubules can contract or expand to impinge on the cells in the pulp in response to thermal stimuli applied on the tooth surface. Hence, dentin of the intact tooth can transform external stimuli into an appropriate message to cells and nerves in the pulp – a feature that is useful clinically to test its vital functions (see Chapter 14). A sensory transducer function triggered by elastic deformation is also in effect to detect overload resulting in reflex withdrawal and sharp transient pain.
Fig. 2.1 The soft tissue of the pulp is surrounded by dentin and enamel or cementum. Inset depicts the interface zone between dentin and pulp.
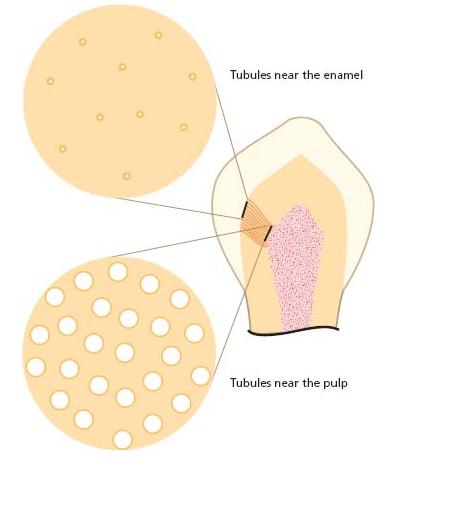
When enamel and cementum are damaged for any reason, the exposed dentinal tubules serve as pathways to the pulp for entry of potentially noxious elements in the oral environment including bacterial macromole-cules, which may provoke inflammation (4). The deeper the injury the more tubules become involved (Fig. 2.2). In the periphery there are about 20 000 tubules per square millimeter, each having a diameter of 0.5 μm. At the pulpal ends the tubular apertures occupy a greater surface area because the tubules converge centrally and become wider (2.5–3 μm) (20). Thus, at the inner surface of dentin there are more than 50 000 tubules per square millimeter. In root dentin, especially towards the apex, the tubules become more widely spaced. Also, in the pulpal portion of root dentin they are thinner and have a smaller diameter (ca. 1.5 μm). There are extensive branches between the tubules that allow intercommunication.
Movement of particulate matter and macromolecules by way of the dentinal tubules may occur not only from the external environment to the pulp but also in the opposite direction. Hence, following injury which has resulted in disruption of the tight junctions that normally hold the odontoblasts together (71), fluid in the pulp may enter the tubules and bring plasma proteins with antimicrobial properties (41).
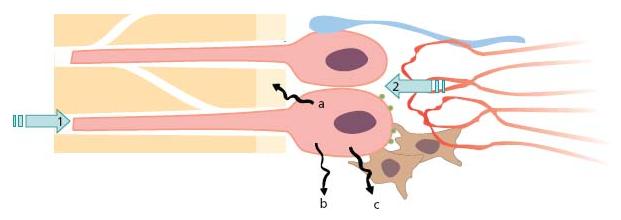
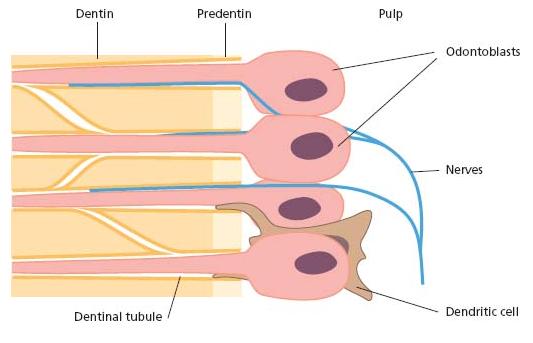
The potential for elements to permeate the dentinal tubules is normally greatly restricted by a variety of tissue structures, including collagen fibers and cellular processes. The odontoblasts normally extend cytoplasmic processes into the tubules. Controversy exists, however, as to how far. While some believe that these processes extend all the way to the enamel or cementum junctions others contend that only the innermost part (0.5–1 mm) of dentin is filled (15). A large number of the tubules also contain nerve terminals. Furthermore, cells belonging to the immunosurveillance system of the pulp extend dendrites into the tubules of the predentin layer (52). Consequently, the space available in the tubules for the transport of particulate matter and macromolecules is normally much smaller than the tubular space per se (61) (Fig. 2.3). This is especially true at their pulpal ends.
Fig. 2.2 Density of dentinal tubules in various portions of the crown region in teeth. It has been estimated that the surface area taken by cross cut tubules is ca. 2–3% in the periphery. Near the pulp the dentinal tubules assume ca. 25% of the surface area (61).
Fig. 2.3 Cellular extensions of odontoblasts, nerves and cells of the immune system (dendritic cells) occupy the pulpal ends of the dentinal tubules.
The odontoblast – a multifunctional cell
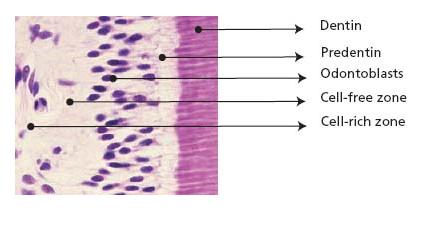
The most recognized function of the odontoblasts is to form and maintain dentin. Like many other tissue-supporting cells, odontoblasts also contribute to host defense. By lining the periphery of the pulp with cellular extensions into dentin they are, thus, in the unique position of being the first cell to encounter and react to noxious elements entering dentin from the oral environment (Fig. 2.4). Upon challenge the odontoblasts generate and release a multitude of molecules that can help to defeat invading microorganisms. The response also gives rise to activation of specific receptors present on adjacent cells, vessels, nerves and on the odontoblast itself (Advanced concept 2.1). Thus, the odontoblasts, together with local resident defense cells and blood-borne invading cells, have a broad repertoire of response patterns and play important roles in activating both innate and adaptive immune responses of the pulp (for reviews see Refs 23, 24) (Fig. 2.5).
Dentin formation
The original odontoblasts, here also termed primary odontoblasts, produce dentin both during tooth development and after completion of root formation. The fact that intratubular cellular processes stay behind makes dentin tubular in nature. Owing to the continued function of the odontoblasts, the pulpal space gradually narrows over time and in old individuals it may become so small that endodontic treatment is difficult.
The odontoblasts may also produce new dentin at an increased rate in response to mild stimuli: e.g. during initial precavitated stages of enamel caries (10); by slowly progressing caries in general (9); or following a shallow preparation for restorative purposes. This type of new dentin has been termed reactionary dentin (67) (see also Core concept 2.1).
Dentinal repair
Following injury or irritation (e.g. by a restorative procedure or rapidly progressing caries), the primary odonto-blasts may die. Because these cells are postmitotic cells, they are unable to regenerate by cell division. New dentin may nevertheless be formed. Such dentinal repair appears to occur through the activity of so-called repairing odontoblasts or secondary odontoblasts. The precursor of these cells is thought to be a population of postnatal stem cells that are present in the pulp tissue proper (22). Following their recruitment and upregulation, a mineralizing matrix is laid down on the dentinal wall. Repair by secondary odontoblasts is also possible against an appropriate wound-healing agent applied to treat direct exposure of the pulp (see Chapter 4). Hence, a new generation of odontoblast-like cells, capable of making new dentin locally, can evolve in the pulp upon injury.
Fig. 2.4 Tissue section stained with hematoxylin and eosin showing dentin, predentin and pulp tissue proper with odontoblasts lining the periphery.
Secondary odontoblasts produce dentin at a rate that is dependent on the extent and duration of the injury. The development of this hard tissue leads to an increase in dentin thickness (Figs 2.6 and 2.7).
It should be noted that dentin formed by secondary odontoblasts is more irregular and amorphous and contains fewer dentinal tubules than primary dentin (11). These tubules will not necessarily be in direct line with the tubules of the primary dentin (Fig. 2.8). Consequently, a complex of primary and reparative dentin becomes less permeable to externally derived matter. It also follows that such dentin is less sensitive to thermal, osmotic and evaporative stimuli (12; see also Chapter 3). The quality of the new hard tissue is not always as good as that of primary dentin. When it is formed rapidly, e.g. following an ischemic injury by dental trauma, it may become highly porous and contain areas filled with soft tissue. Although the pulpal space in radiographs may appear completely obliterated, these areas are large enough to give room for bacterial growth and multiplication in case of an ensuing infectious exposure (Fig. 2.9). Similarly, hard-tissue repair of pulpal wounds may occasionally show gross defects, which makes it highly permeable to bacteria and bacterial elements. Therefore, hard-tissue deposition in the pulp, although adding to the defense potential of the tissue in certain instances, should be viewed as a scar tissue.
Fig. 2.5 The odontoblast has many functions which change during tooth development, maturation and injury of teeth. (a) Sensor: 1. affected from outside by antigens, mechanical forces, thermal gradients; 2. bombarded from inside by circulating hormones, para-crine and autocrine substances. (b) Secretory cell: a. for dentin lay down, b. for maintenance, c. for immune defense. (c) Pain mediator: acting as a transducer between external stimuli and pulpal sensory nerves.
Fig. 2.6 Microphotograph showing hard tissue repair following a cavity preparation (arrow). The circle indicates bulk of new dentin being formed.
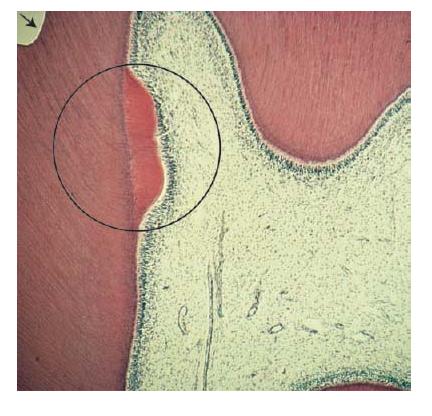
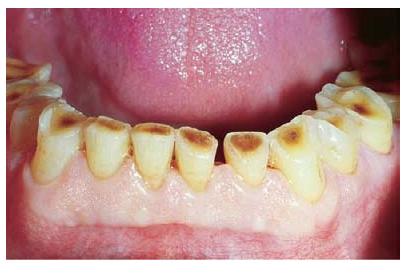
Fig. 2.7 Clinical photograph of anterior lower teeth showing extensive loss of tooth structure due to tooth wear. Reparative dentin formed in the pulp has prevented direct exposure of the tissue to the oral environment.
Nerves
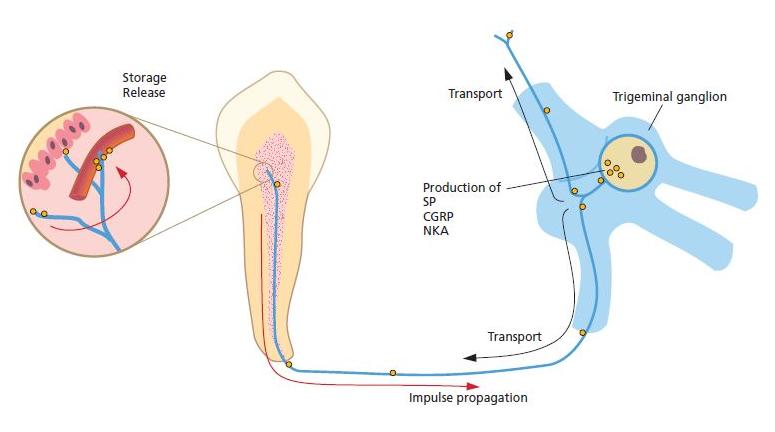
Pulpal nerves monitor painful sensations. By virtue of their peptide content they also play important functions in inflammatory events and subsequent tissue repair (Fig. 2.10). In addition, they control dentin formation (Fig. 2.10).
Fig. 2.8 Tissue section of an interface zone between primary dentin and reparative dentin as indicated by arrows. Note that the dentinal tubules are less numerous in the secondary dentin than in the primary dentin to the left. Also, few of the tubules are in direct line with those of the primary dentin, thus making the entire complex less permeable. Pulpal tissue and nuclei of pulp cells are to the right. (Courtesy of Dr Lars Bjørndal and with permission of Caries Research, Karger.)
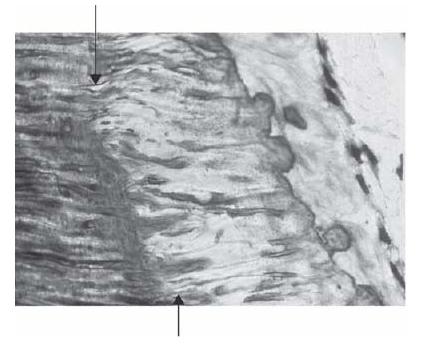
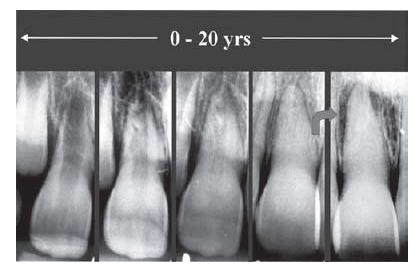
Fig. 2.9 Series of radiographs of a tooth in a patient who suffered a luxation injury at a young age. Hard tissue is successively deposited in the pulp. Arrow indicates change in status between the 15-year and 20-year follow-up radiographs. In the radiograph to the right, a periapical radiolucency is seen, suggesting pulpal infection. (From Robertson et al. (65) with permission of the Journal of Endodontics.)
There are two types of nerve fiber that mediate the sensation of pain: A-fibers conduct rapid and sharp pain sensations and belong to the myelinated group, whereas C-fibers are involved in dull aching pain and are thinner and unmyelinated. The A-fibers, mainly of the A-delta type, are preferentially located in the periphery of the pulp, where they are in close association with the odon-toblasts and extend fibers to many but not all dentinal tubules. The C-fibers typically terminate in the pulp tissue proper, either as free nerve endings or as branches around blood vessels (56). The nature of A- and C-fibers and their respective roles in pain transmission are described in Chapter 4.
Fig. 2.10 A large portion of the sensory fibers, including C-fibers and some A-delta fibers, contain vasoactive neuropeptides such as calcitonin gene-related peptide (CGRP), substance P (SP) and neurokinin A (NKA). The neuropeptides are produced in the trigeminal cell bodies and are transported via axonal flow to the nerve terminals in the pulp, where they are stored. In addition to their effect on pulpal blood flow and vessel permeability, SP and CGRP exert stimulatory effects on the growth of pulpal cells, such as fibroblasts and repairing odontoblasts. They are also active in the recruitment of immunocompetent cells in response to bacterial exposures.
Nerves belonging to the autonomic nervous system, such as sympathetic vasoconstrictor fibers, are also present (48). They enter the pulp together with blood vessels and sensory axons. Histochemically, they can be traced in the pulp via their content of noradrenaline and neuropeptide Y. Upon release, these substances result in contraction of the smooth-muscle sphincters in arteries and small arterioles apical to and within the pulp (58).
Both sensory and sympathetic nerves stimulate dentin formation as evidenced by reduced dentin formation in the absence of sensory nerves and after sympathectomy, respectively (32). Sensory and sympathetic nerves also interact in pulpal inflammation. For example, an intact innervation is significant for recruitment and activation of cells of the immune system (see Key literature 2.1).
As yet, there is little evidence that parasympathetic vasodilator blood flow control plays an important role in the local function and defense of the pulp.
Vascular supply
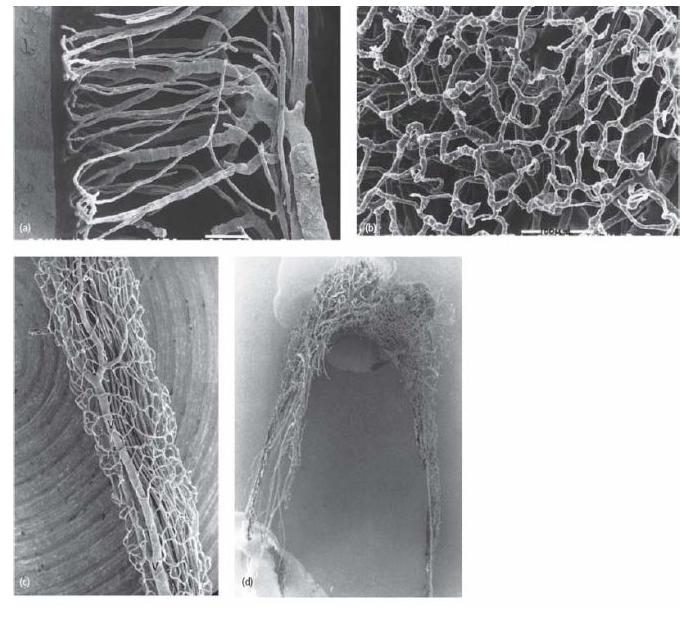
Current knowledge of the vascular architecture of the pulp has been influenced greatly by the use of the micro-vascular resin cast method (Fig. 2.11) (68). This technique allows resin to fill up even the smallest capillaries of the pulp. A vascular cast is then obtained, which, following corrosion of surrounding tissue structures, can be examined in the scanning electron microscope.
In all developmental stages the crown pulp shows a larger vascular network than the root pulp. In more central portions of the pulp the vascular network is less dense than in peripheral pulp. Anastomosis between incoming and outgoing blood vessels has been observed in the central pulp of adult animal teeth (40) and seems to be more frequent in the apical pulp than in the crown pulp (38). Shunt connections between supplying and draining pulpal vessels have also been found just outside the apical foramen in the periodontal ligament (69). It is reasonable to assume that these shunts provide control of blood perfusion through the pulpal tissue. Hence, in the case of a local inflammatory event causing increased resistance to pulpal blood flow, arteriovenous shunts may come into play and redirect incoming blood.
Fig. 2.11 Series of microphotographs of the vascular network in the pulp of teeth. (a) In the young tooth of dogs there is a dense terminal capillary network in the pulp–dentin border zone. (b) The superficial capillary network in the odontoblast region in a view perpendicular to the pulpal surface. (c) Blood vessels in the distal root canal of a mature dog premolar. The superficial capillaries drain directly into large venules (V). In the mature tooth, continuous dentin formation and narrowing of the pulp cavity lead to remodeling of the vascular tree. (d) The vascular network of an adult human tooth. With a narrow apical foramen, the number of arterioles is reduced to 5–8 and venules to 2–3 (40). The number of main vessels, arterioles and venules in the central pulp is also reduced and the typical hairpin loops of the terminal capillary network become less pronounced. The detailed vascular architecture of the pulp is similar in cat, dog and human teeth. (Courtesy of Dr K. Takahashi.)
Lymphatics
Both morphological and functional studies in animals show the existence of lymphatic vessels in the pulp (8, 26). These vessels are important to adjust for increased colloid osmotic pressures exerted by proteins and macro-molecules accumulating extracellularly in inflamed areas. Another important function is to serve as pathways to the regional lymph nodes for antigen-presenting cells.
Immune defense
The dentin–pulp complex is uniquely organized to offset microbial threats from caries and other breaches of the outer hard tissue encasement of the tooth to the oral environment. While permeable to bacterial elements by virtue of the dentinal tubules, dentin nonetheless carries out an important filter function of significance to the pulp’s immune defense. Primarily two mechanisms account for this effect: (i) the peripherally directed flow of dentinal fluid, and (ii) the absorbance of bacteria and bacterial macromolecules to the inner walls of the tubules (63). Thereby, dentin is able to temper exposures of noxious elements to the pulp, allowing it to adapt and organize an effective immune defense response.
Constituents making up the innate immune defense of the pulp include resident tissue cells, viz. odontoblasts and immune cells, nerves and vessels (Fig. 2.12). The basal set up of immune cells is limited to antigen-presenting cells (APCs), macrophages and T- lymphocytes (T-cells) of the memory type (23). Neither B-cells nor mast cells are residents of the normal pulp and will not appear in the tissue unless there is an inflammatory event.
APCs in the normal pulp are of two types. One has a pronounced dendritic configuration and belongs to the family of dendritic cells (DCs). The other is of the monocyte/macrophage lineage. Both constitutively carry class II MHC molecules on their cell surface. This molecule, found on all cells capable of antigen presentation, is a gene product of the major histocompatibility complex (MHC) and acts as a stimulatory molecule in T-cell activation both locally and in the regional lymph nodes.
Fig. 2.12 Constituents of primary significance in the defense of the pulp against foreign substances, including bacterial elements, make up the innate “first line of defense”.
The DCs in the pulp are strategically positioned in the periphery of the tissue, where foreign antigens are most likely to enter (Figs 2.12 and 2.13). Here, they compete for available space with the odontoblasts and make contact with these cells via their cytoplasmatic processes (52). Pulpal DCs are also in close proximity with nerve tissue elements and blood vessels in the paraodonto-blastic region, suggesting interactive potentials (53, 54).
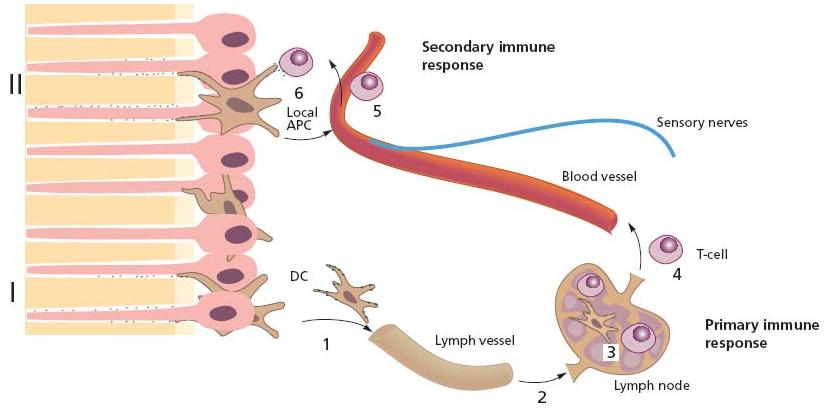
The primary function of DCs is to alert the immune system for an effective subsequent elimination, and not to directly combat invading microorganisms. In peripheral sites, like the pulp, they occur in an immature state. Maturation to become professional APCs (capable of activating T-cells that have not been exposed to antigen before, so-called naïve T-cells) comes from capturing incoming foreign antigens. Upon exposure they usually start migrating to the regional lymph nodes. Once there, fragments of the antigen bound to the class II MHC molecule will be shown to the appropriate naïve T-cells that become activated in a primary immune response (Fig. 2.14). DCs are particularly effective in microbial sensing, capturing and processing foreign antigens and are thus a key initiator of the adaptive immune response.
Fig. 2.13 Tissue section showing dendritic cells (stained brown) within the odontoblastic and subodontoblastic layer. Immunohistochemical staining was carried out with OX6-antibody, which is a marker for class II MHC molecules.
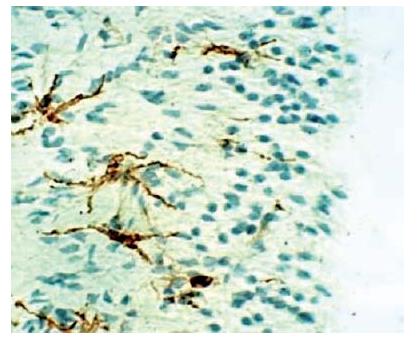
Fig. 2.14 Antigen-specific T-cells are developed in the pulp following primary (I) and secondary (II) antigen exposures along dentinal tubules. Dendritic cells (1 in figure) capture protein antigen for processing to peptide fragments and carry (2) and present peptide fragments in the context of the class II molecules on their cell surface to naïve T-cells in the regional lymph nodes (3: primary immune response). Following clonal expansion, these cells enter the circulation (4 in figure). Following their patrolling of tissues as memory T-cells, they may participate in secondary immune responses at local sites, e.g. in the pulp (5 in figure), if exposed to the appropriate antigen by local APC (6 in figure). This route constitutes adaptive pathogen-specific immunity.
The class II molecule-expressing macrophages are distributed in a remarkably high number in the pulp and seem to form a dense network together with pulpal DCs (33, 34). Like other connective tissues, macrophages in the pulp are heterogeneous in terms of phenotype and function. While there are those serving in local antigen presentation, there is also a large population of non-class II molecule-expressing, resident macrophages (histio-cytes), primarily located perivascularly with primary functions in phagocytosis.
Basal maintenance
Blood flow
It is assumed that odontoblasts and nerve endings, especially during tooth development, have a high energy demand. This may also be true in mature teeth. Hence, although there is limited collateral circulation, the peripheral pulp is well vascularized (see Fig. 2.11) The blood flow through the young adult pulp during resting conditions is relatively high compared with that of other oral tissues (50). In the adult dog, blood flow per 100 g of tissue is ca. 40 ml/min in teeth with a fully formed apex. By comparison, in the gingiva it is ca. 30 ml/min. The dense capillary network in peripheral pulp also allows filtration of fluid from the blood vessels, thereby supplying the dentinal tubules with fluid. In old teeth blood perfusion becomes successively lower.
Local control
The level of the resting blood flow in the pulp is to a great extent controlled by the neuropeptides substance P (SP) and calcitonin gene-related peptide (CGRP). Both CGRP and SP maintain a continuous relaxation of feeding arterioles (7). This continuous influence on the blood supply to the pulp depends on a basal release of the peptides without apparent nerve activation.
Nitric oxide (NO) – a short-lived gas molecule that is produced enzymatically in the endothelial cell lining of vessels – also serves to maintain a physiological blood perfusion of the pulp (37). It has a powerful vasodilator action and, unlike neuropeptides, causes relaxation of the draining venules in the pulp under physiological conditions (7) (see Advanced concept 2.2).
Remote control
The regulatory control of pulpal blood flow also involves autonomic nerves. This remote system influences blood circulation in the pulp as well as in adjacent tissues within the same innervation territory.
Although parasympathetic vasodilator nerves do not seem to play a significant role, there is firm evidence for sympathetic vasoconstrictor control in the dental pulp. The system does not seem to be active tonically and may not support local moment-to-moment demands of the tissue. However, physical and mental stresses trigger sympathetic vasoconstriction in the oral region, includin/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses