Inflammation and Repair
Margaret J. Fehrenbach and Joan Andersen Phelan
After studying this chapter, the student will be able to:
1. Define and pronounce each of the words in the vocabulary list for this chapter.
2. List the classic signs of inflammation that occur locally at the site of inflammation.
3. List and describe the major systemic signs of inflammation.
4. List and describe the microscopic events of the inflammatory process.
5. Describe the microscopic events associated with each of the local signs of inflammation.
6. List the white blood cells that are involved in acute inflammation and describe how each is involved.
7. Describe the differences between acute and chronic inflammation.
8. Define and contrast hyperplasia, hypertrophy, and atrophy.
9. Describe the microscopic events that occur during mucosal tissue repair
10. Describe the microscopic events that occur during healing in bone.
11. Describe and contrast healing by primary intention, healing by secondary intention, and healing by tertiary intention.
12. List local and systemic factors that can impair healing.
13. Describe and contrast internal and external tooth resorption.
14. Describe and contrast attrition, abrasion, and erosion.
15. Describe the pattern of erosion seen in bulimia.
16. Describe the relationship between bruxism and abrasion.
17. Describe the cause, clinical features, and treatment of each of the following: aspirin and phenol burns, electric burn, traumatic ulcer, frictional keratosis, linea alba, and nicotine stomatitis.
18. Describe the clinical features, cause (when known), treatment, and microscopic appearance of each of the following: traumatic neuroma, postinflammatory melanosis, oral and labial melanotic macule, solar cheilitis, mucocele, ranula, necrotizing sialometaplasia, pyogenic granuloma, peripheral giant cell granuloma, chronic hyperplastic pulpitis, and irritation fibroma.
19. Describe the difference between a mucocele and a ranula.
21. Describe the difference between acute and chronic sialadenitis.
22. Describe the clinical features, radiographic appearance, and microscopic appearance of a periapical abscess, a periapical granuloma, and a radicular (periapical) cyst.
Inflammation, immunity, and repair are the body’s responses to injury. Inflammation allows the human body to eliminate tissue injured by trauma or infection, to contain injuries, and to begin the process of healing. This chapter begins with a description of the inflammatory response, tissue regeneration, and repair, and continues with a description of oral lesions that occur in response to injury. Many of these lesions are quite common and are likely to be encountered when the dental hygienist examines the hard and soft tissues of the oral cavity. Lesions that occur as a result of destruction through activity of the immune responses are described in Chapter 3. Lesions that occur as a result of infection are included in Chapter 4.
Inflammation
Thus transitional stages exist during which the response is changing from one type of inflammation to the next and from an innate inflammatory response to an immune response (see Chapter 3). In addition, an acute inflammatory response may be superimposed over a chronic inflammatory response. On occasion, an overwhelming inflammatory response may lead to further injury. Repair of the tissue occurs only if the persistent source of injury is removed.
Microscopic Events of Inflammation and Clinical Signs
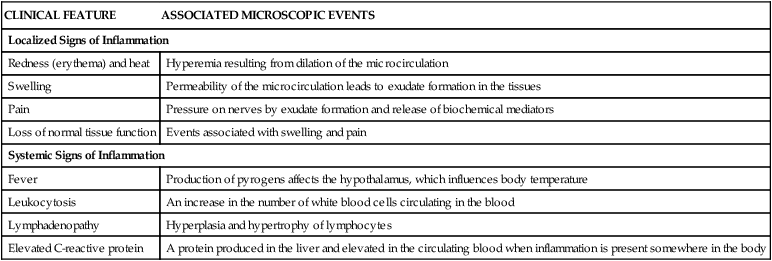
Microscopic events occur within the injured tissues during both acute and chronic inflammation. These events cause changes that can be observed clinically. The local clinical changes at the site of injury are called the classic (cardinal) signs of inflammation: redness, heat, swelling, pain, and loss of normal tissue function (Table 2-1). In addition, systemic signs of inflammation may be present when the response is more extensive; these signs are discussed later in this chapter.
TABLE 2-1
Local and Systemic Clinical Signs of Inflammation and Associated Microscopic Events
| CLINICAL FEATURE | ASSOCIATED MICROSCOPIC EVENTS |
| Localized Signs of Inflammation | |
| Redness (erythema) and heat | Hyperemia resulting from dilation of the microcirculation |
| Swelling | Permeability of the microcirculation leads to exudate formation in the tissues |
| Pain | Pressure on nerves by exudate formation and release of biochemical mediators |
| Loss of normal tissue function | Events associated with swelling and pain |
| Systemic Signs of Inflammation | |
| Fever | Production of pyrogens affects the hypothalamus, which influences body temperature |
| Leukocytosis | An increase in the number of white blood cells circulating in the blood |
| Lymphadenopathy | Hyperplasia and hypertrophy of lymphocytes |
| Elevated C-reactive protein | A protein produced in the liver and elevated in the circulating blood when inflammation is present somewhere in the body |
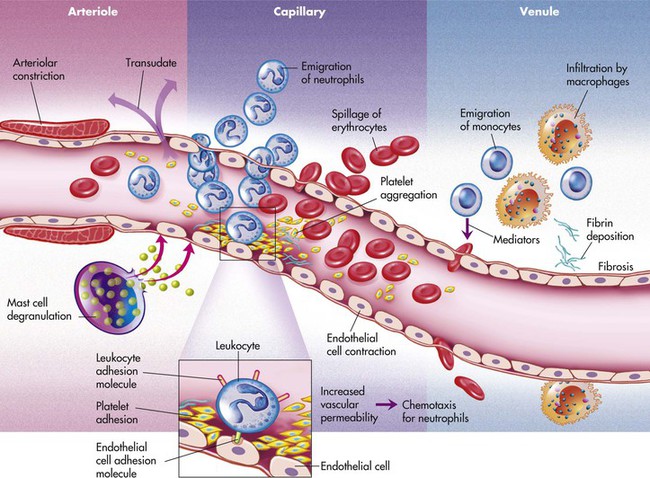
The microscopic events of inflammation involve the small blood vessels or microcirculation. These include arterioles, capillaries, and venules in the area of injury as well as red blood cells, white blood cells, and chemicals in the body called biochemical mediators (Figure 2-1). Normally, blood, and the cells it contains, flows through the microcirculation. Exchange of oxygen and nutrients needed for the health of the surrounding tissue occurs as plasma fluid passes between the endothelium lining the vessel walls of the arterioles and capillaries. Plasma is the fluid component of blood in which the blood cells are suspended; it is composed mainly of water and proteins. Normally most of the plasma that leaves the microcirculation reenters the circulation through the venules. The lymphatic vessels carry away any excess plasma that does not reenter the blood vessels.
During inflammation these normal events undergo a change, with underlying microscopic events proceeding faster than the visible clinical changes. The sequence of events is shown in Box 2-1.
While hyperemia is occurring, the permeability of the vessels of the microcirculation also increases; the blood vessels become “leaky.” The endothelial cells contract and spaces form between the cells. As a result, plasma fluid with a low protein content that contains no cells passes between the endothelial cells and enters the tissue. This fluid is called a transudate and is the same type of fluid that normally moves from the microcirculation to the tissues to supply oxygen and nutrients. The loss of fluid within the microcirculation leads to increased blood viscosity. The blood becomes thicker and cannot flow as easily. This eventually results in decreased flow through the microcirculation. As the blood flow slows down, the red blood cells begin to pile up in the center of the blood vessels, and the white blood cells are displaced to the periphery of the blood vessels. This movement of the white blood cells to the periphery is called margination. The white blood cells are now in position to adhere themselves to the inner walls of the injured blood vessels, which have become “sticky” because of specific factors on the surfaces of the cells. This lining of the walls by white blood cells is called pavementing (Figure 2-2).
After pavementing the vessel walls, the white blood cells begin to escape from the blood vessels, along with more fluid, and enter the injured tissue. This process by which the white blood cells escape from the blood vessels is called emigration. Emigration occurs as a result of opening of the cellular junctions of the endothelial cells lining the blood vessels; these cells contract in size in response to biochemical mediators. As the white blood cells (primarily neutrophils) emigrate through the blood vessel walls and surrounding basement membrane, they further increase the permeability of the microcirculation and allow larger molecules and other cells to escape. The fluid that now flows into the injured tissues is called an exudate. This fluid contains cells and a higher concentration of protein molecules than in a transudate. The presence of transudate and exudate in the injured tissue helps to dilute injurious agents that may be present and carries injurious agents through the lymphatic vessels to the lymph nodes, where an immune response is stimulated (Chapter 3).
As transudate escapes into the tissue, excess fluid collects in the fibrous connective tissue at the site. This excess fluid in the interstitial space is called edema and results in localized enlargement or swelling of the tissue, another clinical sign of inflammation (Figure 2-3). If the swollen tissue area is injured further, exudate may flow out of the tissue as either a thin, clear fluid (serous exudate) or as a thick, white-to-yellow pus that contains tissue debris and many white blood cells (purulent exudate). An abscess is a collection of purulent exudate that has accumulated in a cavity formed by the tissue.
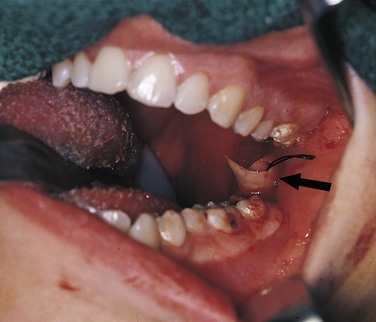
The formation of exudate may be so excessive that it interferes with repair of the tissue. The injured tissue may allow the excess exudate to drain by formation of a drainage passage that bores through the tissue, allowing drainage to the outside. This channel through the tissue is called a fistula or fistulous tract; it is formed at the expense of healthy, functioning tissue in the area that is lost as the tissue undergoes necrosis (Figure 2-4). In some cases, excessive exudate in damaged tissue must be drained mechanically by making an incision in the surface of the swollen area and, often, by placing a drainage tube in the site of the incision (Figure 2-5). This procedure of incision and drainage may be accompanied by the administration of an antibiotic and medication to reduce inflammation.
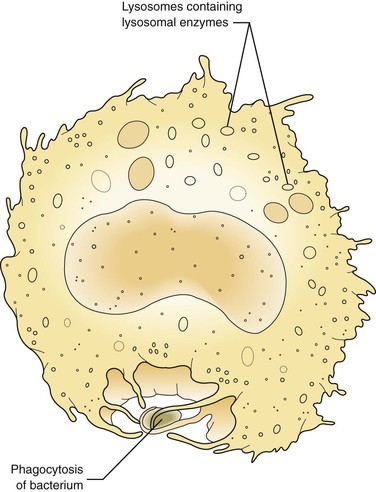
At first these cells try to wall off the site of the injury from the surrounding healthy tissue. Later, in the injured tissue, the white blood cells also try to remove foreign substances from the site by ingesting and then digesting them, thus undergoing phagocytosis (Figure 2-6). The foreign substances may include pathogenic microorganisms or tissue debris. The presence of these substances interferes with the repair process; therefore they must be removed for the inflammation to resolve and any necessary tissue repair to proceed.
White Blood Cells in the Inflammatory Response
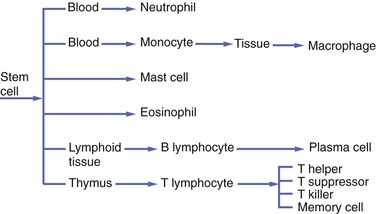
Emigration of white blood cells (leukocytes) from the blood vessels into the site of injury and subsequent chemotaxis and phagocytosis are important components of the process of inflammation. Two types of white blood cells are initially involved in the inflammatory response: (1) neutrophils and (2) monocytes circulating in the blood, which become macrophages in tissue. Neutrophils are also called polymorphonuclear leukocytes because they are the most prevalent of the white blood cells that contain a multilobed nucleus. However, this term also includes other white blood cells; therefore neutrophil is a more specific name for this cell. Other cells within the blood and tissue, such as lymphocytes and plasma cells, eosinophils, and mast cells, participate in both inflammatory and immune responses. The immune response and the involvement of these cells are discussed in Chapter 3.
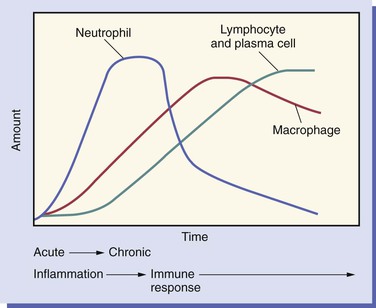
As inflammation begins and continues over the 2 weeks after an injury, changes take place in the relative numbers of white blood cell populations present in the tissue (Figure 2-7).
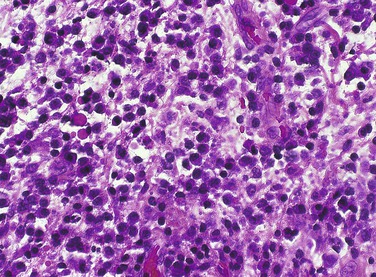
The neutrophil is the first type of white blood cell to arrive at the site of injury and is the most common inflammatory cell present during acute inflammation (Figure 2-8). The second type of white blood cell to arrive at the site of injury within the tissue is the macrophage. This cell, when circulating in the blood, was called a monocyte. When it leaves the microcirculation and enters the tissue it becomes a macrophage. As inflammation continues, the number of neutrophils decreases. If the injury persists and chronic inflammation occurs, macrophages, lymphocytes, and plasma cells replace the neutrophils and become the most prevalent white blood cells present in the tissue (Figure 2-9).
Neutrophils
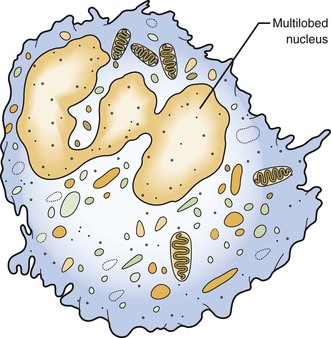
As discussed, the neutrophil is the first type of white blood cell recruited into the area of injury in response to chemotactic factors. The main function of the neutrophil is phagocytosis of substances such as pathogenic microorganisms and tissue debris. Microscopically, neutrophils possess a multilobed nucleus, which is why they are called polymorphonuclear leukocytes, and a granular cytoplasm that contains lysosomal enzymes (Figure 2-10). Lysosomal enzymes contained within vacuoles in the cell cytoplasm destroy substances after the cell has engulfed them. Removal of these substances from the site of injury is necessary to allow the process of repair. Neutrophils die shortly after phagocytosis; as a result, lysosomal enzymes and other damaging cellular substances that were meant only for intracellular destruction of foreign substances are released from the cells. This can cause further tissue damage to the site when large numbers of neutrophils die.
Normally, neutrophils constitute 60 to 70 percent of the entire white blood cell population. Like all white blood cells, neutrophils are derived from stem cells in the bone marrow (Figure 2-11). Stem cells are undifferentiated cells in the spongy tissue that is found in the center of certain long and flat bones, such as the bones of the pelvis and sternum. Neutrophils are produced throughout life and are mobile cells.
Macrophages
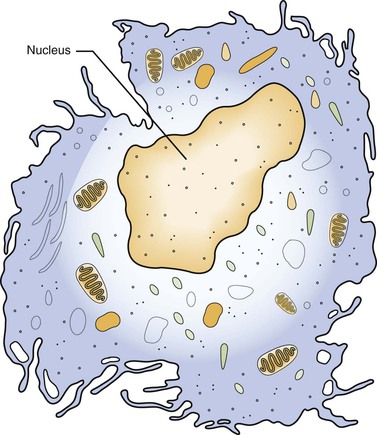
The monocyte is the second type of white blood cell to emigrate from the blood vessels into the injured tissue, where it becomes a macrophage. Like neutrophils, monocytes are derived from stem cells in the bone marrow (see Figure 2-11). As a macrophage, it responds to chemotactic factors, is capable of phagocytosis, is mobile, and has lysosomal enzymes in its cytoplasm that assist in the destruction of foreign substances within the cell.
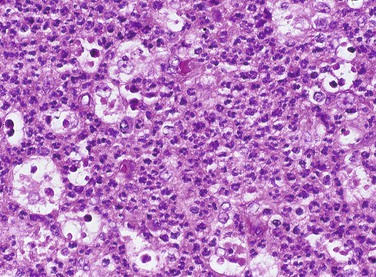
The macrophage is larger than its monocytic precursor. It constitutes 3 to 8 percent of the entire white blood cell population. Microscopically, it has a single round nucleus and does not have granular cytoplasm (Figure 2-12). The macrophage has a somewhat longer life span than the neutrophil. In addition to its role in phagocytosis during inflammation, the macrophage is also an important cell in the immune response (see Chapter 3).
Biochemical Mediators of Inflammation
Complement System
The complement system is composed of a series of plasma proteins that are activated in a cascading fashion, with one protein activating the next in the series. Components of the complement system function during both inflammation and immunity. Complement components can cause mast cells to release granules from their cytoplasm that contain the biochemical mediator histamine and other mediators into the surrounding tissue. Mast cells are active during certain inflammatory reactions. They are normally located in large numbers in the loose connective tissue of the skin and mucosa. When histamine is released from mast cells it causes an increase in vascular permeability and vasodilation (see Figure 2-1). Other components of the complement system can cause cell death by creating holes in the cell membrane (cytolysis). Complement proteins can also attach to the surface of bacteria, stimulating white blood cells to phagocytize them, a process called opsonization.
Systemic Manifestations of Inflammation
In addition to the local features of inflammation, four major systemic signs may also occur: (1) fever, (2) an increase in the number of white blood cells (leukocytosis), (3) enlargement of lymph nodes (lymphadenopathy), and (4) elevated C-reactive protein (see Table 2-1).
Lymphadenopathy
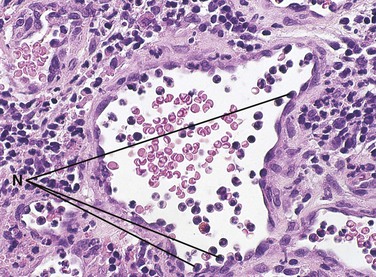
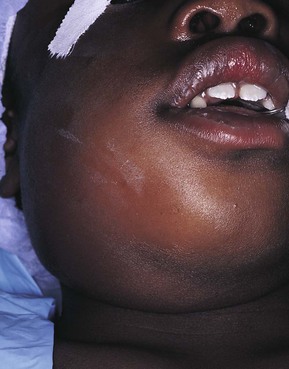
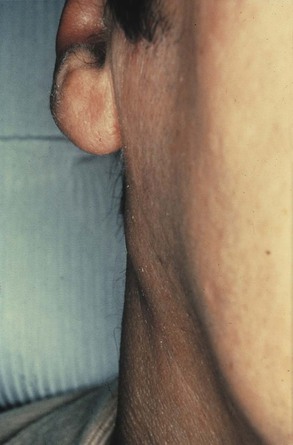
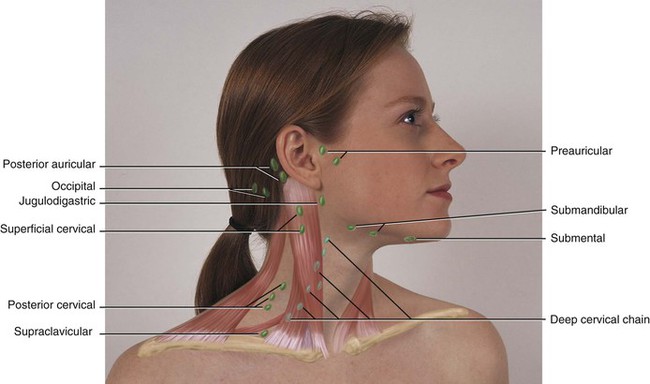
During the inflammatory process the lymph nodes enlarge. This enlargement of the lymph nodes is referred to as lymphadenopathy (Figure 2-13). The enlarged lymph node or nodes, if located superficially, can be palpated as a mass or masses in the area of inflammation and possibly along the associated lymphatic drainage route (Figure 2-14). When palpated, the involved node feels firmer and larger than usual and may also be tender. Deeper lymph nodes may also be enlarged, but these cannot be palpated during an examination.
Lymphadenopathy results from changes in the lymphocytes that reside in the lymph node. Lymphocytes are white blood cells that mature in lymphoid tissue. They are the main white blood cells of the immune response. Lymphocytes travel from the lymph node to the tissues through the circulation where they are involved in the immune response. The role and maturation of lymphocytes is described in detail in Chapter 3. The changes in the lymphocytes cause the change in size of the lymph nodes. These changes include an increase in the number of cells (hyperplasia), resulting from increased cell division, and an enlargement of individual cells (hypertrophy), resulting from cellular maturation. These changes in the lymphocyte population usually occur during chronic inflammation. The lymphoid tissue in Waldeyer’s ring (the palatine, lingual, and pharyngeal tonsillar tissue) may also undergo these changes.
Reactive Tissue Responses
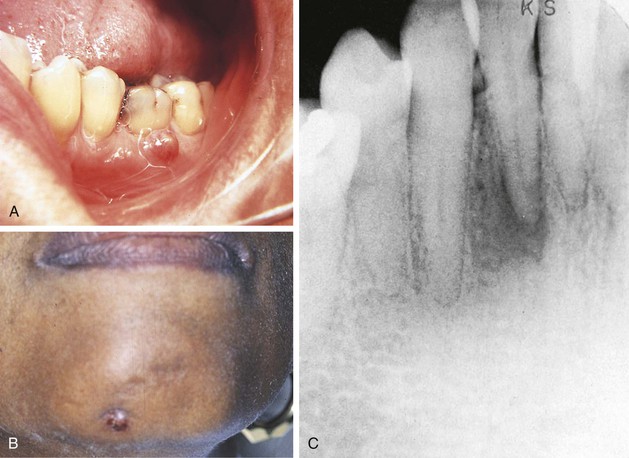
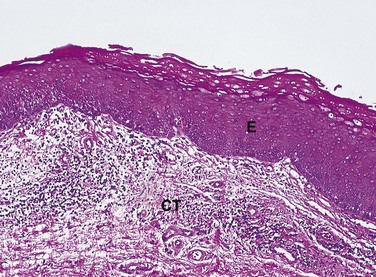
The cells in a tissue or organ may respond to injury by undergoing an adaptive response such as hyperplasia, hypertrophy, or atrophy. Hyperplasia is defined as an increase in the number of cells in a tissue or organ, with a consequent increase in size of the tissue or organ, in response to conditions that cause cellular stress. Pathologic hyperplasia frequently occurs in oral tissues. In the oral cavity an increase in the number of epithelial cells and an increased thickness of the epithelium commonly occur in response to chronic injury (Figure 2-15). Normally, as surface epithelial cells are lost, division of deeper basal epithelial cells increases to replace the lost cells. With hyperplasia, the production of new cells exceeds the original number of cells lost; thus the epithelium becomes thickened, and the tissue appears paler or whiter.
Regeneration and Repair
Microscopic Events during Repair
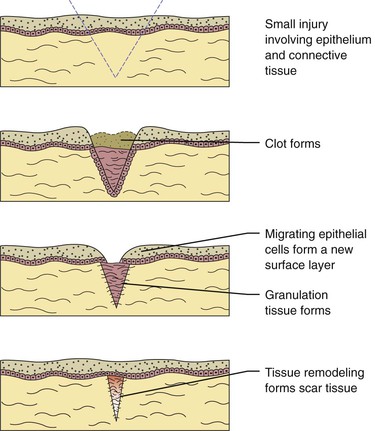
After an injury occurs microscopic events occur in both the epithelium and connective tissue (Figure 2-16). These events are different for each of these tissue types, but occur almost simultaneously and are dependent on each other for optimal healing. If the source of the injury is removed, the repair process for both types of tissues is usually completed within 2 weeks. The repair process is slightly different in the oral cavity than in skin because mucosal tissues are moist and a scab does not form. There are three phases to the repair process that occur during these 2 weeks: (1) inflammation, (2) proliferation, and (3) maturation.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


 )
) )
) )
) )
) )
) )
) )
) )
) )
) )
) )
) )
) )
) )
)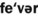 )
) )
) )
)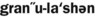 )
)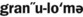 )
) )
) )
) )
) )
) )
)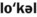 )
) )
) )
) )
) )
) )
) )
) )
) )
) )
) )
) )
) )
)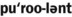 )
)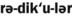 )
) )
)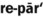 )
)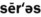 )
) )
) )
) )
)