Dental Ceramics
What Are Ceramics?
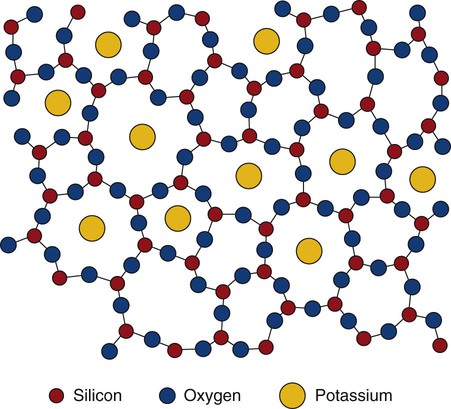
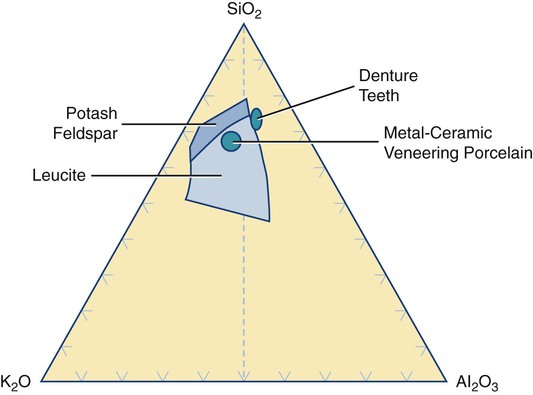
Dental ceramics are nonmetallic, inorganic structures, primarily containing compounds of oxygen with one or more metallic or semi-metallic elements (aluminum, boron, calcium, cerium, lithium, magnesium, phosphorus, potassium, silicon, sodium, titanium, and zirconium). Many dental ceramics contain a crystal phase and a silicate glass matrix phase. Their structures are characterized by chains of (SiO4)4− tetrahedra in which Si4+ cations are positioned at the center of each tetrahedron with O− anions at each of the four corners (Figure 18-1). The resulting structure is not close-packed and it exhibits both covalent and ionic bonds. The SiO4 tetrahedra are linked by sharing their corners. They are arranged as linked chains of tetrahedra, each of which contains two oxygen atoms for every silicon atom. The primary structural unit in all silicate structures is the negatively charged silicon-oxygen tetrahedron (SiO4)4−. It is composed of a central silicon cation (Si4+) bonded covalently to four oxygen anions located at the corners of a regular tetrahedron. For feldspathic veneering porcelains, alkali ions such as sodium or potassium occupy sites that allow them to bond to electrons from unbalanced oxygen ions (see Figure 18-1). Alkali cations such as potassium or sodium tend to disrupt silicate chains and increase the thermal expansion of these glasses. The expansion coefficient (TEC) can be further increased by including crystalline particles such as tetragonal leucite (K2O•Al2O3•·4SiO2 or KAlSi2O6), whose TEC ranges from 22 to 30 × 10−6/K. Two of the primary phase fields (potash feldspar and leucite) that are found in commercial feldspathic veneering ceramics are shown in a ternary section of the K2O•Al2O3•SiO2 phase diagram in Figure 18-2. Sanidine (KAlSi3O8), a potassium aluminosilicate phase, exists at high temperatures although it may be retained on cooling in the form of monoclinic crystals.
VITABLOCS Mk II is the only known dental ceramic with sanidine as the primary crystal phase. The glass matrix phase in these porcelains is formulated from one or more forms of the mineral feldspar (KAlSi3O8, NaAlSi3O8, and CaAl2Si2O8). Many of the veneering ceramics (also called porcelains) are derived from potash feldspar (K2O•Al2O3•6SiO2 or KAlSi3O8), although some may be based on soda feldspar or a combination of both types. Compositions of some dental ceramics are listed in Table 18-1. In industry, the term porcelain is generally associated with ceramics produced with a significant amount of kaolinite [Al2Si2O5(OH)4 or Al2O3•2SiO2•2H2O]. Kaolinite is a form of kaolin, which is a type of clay. None of the modern low-fusing or ultralow-fusing porcelains contains any clay product such as kaolinite. However, it may be used in the formulations for high-fusing porcelain and ceramic denture teeth (see Figure 18-2). Thus, these ceramics are technically not porcelains and they can be considered a type of glass (e.g., leucite glass, fluorapatite glass, or feldspathic glass). However, until the international community sees the need to change our terminology from porcelain to glass, we will continue to use the term porcelain.
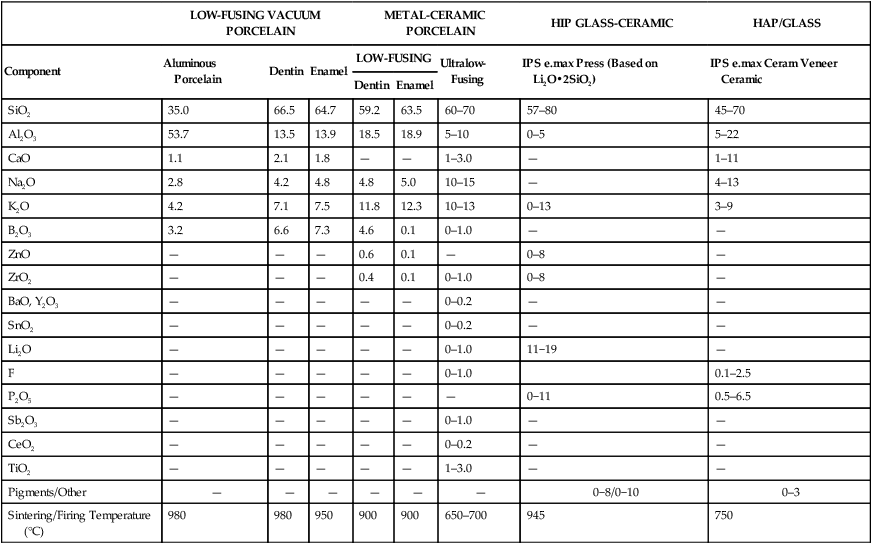
TABLE 18-1
Composition (Percentage by Weight) of Selected Ceramics
| LOW-FUSING VACUUM PORCELAIN | METAL-CERAMIC PORCELAIN | HIP GLASS-CERAMIC | HAP/GLASS | |||||
| Component | Aluminous Porcelain | Dentin | Enamel | LOW-FUSING | Ultralow-Fusing | IPS e.max Press (Based on Li2O•2SiO2) | IPS e.max Ceram Veneer Ceramic | |
| Dentin | Enamel | |||||||
| SiO2 | 35.0 | 66.5 | 64.7 | 59.2 | 63.5 | 60–70 | 57–80 | 45–70 |
| Al2O3 | 53.7 | 13.5 | 13.9 | 18.5 | 18.9 | 5–10 | 0–5 | 5–22 |
| CaO | 1.1 | 2.1 | 1.8 | — | — | 1–3.0 | — | 1–11 |
| Na2O | 2.8 | 4.2 | 4.8 | 4.8 | 5.0 | 10–15 | — | 4–13 |
| K2O | 4.2 | 7.1 | 7.5 | 11.8 | 12.3 | 10–13 | 0–13 | 3–9 |
| B2O3 | 3.2 | 6.6 | 7.3 | 4.6 | 0.1 | 0–1.0 | — | — |
| ZnO | — | — | — | 0.6 | 0.1 | — | 0–8 | — |
| ZrO2 | — | — | — | 0.4 | 0.1 | 0–1.0 | 0–8 | — |
| BaO, Y2O3 | — | — | — | — | — | 0–0.2 | — | — |
| SnO2 | — | — | — | — | — | 0–0.2 | — | — |
| Li2O | — | — | — | — | — | 0–1.0 | 11−19 | — |
| F | — | — | — | — | — | 0–1.0 | 0.1–2.5 | |
| P2O5 | — | — | — | — | — | — | 0−11 | 0.5–6.5 |
| Sb2O3 | — | — | — | — | — | 0–1.0 | — | — |
| CeO2 | — | — | — | — | — | 0–0.2 | — | — |
| TiO2 | — | — | — | — | — | 1–3.0 | — | — |
| Pigments/Other | — | — | — | — | — | — | 0−8/0−10 | 0–3 |
| Sintering/Firing Temperature (°C) | 980 | 980 | 950 | 900 | 900 | 650–700 | 945 | 750 |
Ceramics are composed of metallic and nonmetallic elements that form crystalline and/or noncrystalline compounds. They may form binary compounds such as alumina (Al2O3) and zirconia (ZrO2) by the bonding of metals, which release their positive valence electrons to nonmetals that can accept or share electrons (negative ions). The free energy for bonding of positive metal ions to negative nonmetal ions must be sufficiently low so that the metallic ions preferentially attract nonmetallic ions rather than their own ions or other positive ions. Molecules with one oxygen atom (such as Na2O, K2O, or CaO) are useful in dental porcelain as fluxes. They may also act as opacifiers. Molecules that contain three oxygen atoms for every two other atoms (such as Al2O3) are used as stabilizers. They are also added as crack blockers or toughening crystals. Silica (SiO2) is the main glass-forming structure used in all dental veneering ceramics. All silicates of dental interest are derived from silica tetrahedral structures that can be linked as chains, double chains, or three-dimensional distributions of tetrahedra. Fluxing cations such as K+ or Na+ neutralize the negative charges of the silicate backbones and disrupt the continuity of silicate networks (see Figure 18-1), leading to lower sintering temperatures and increased coefficients of thermal expansion.
Multicomponent or mixed oxide structures may also be useful for dental applications. Three examples of this class of ceramics include MgO•Al2O3 (spinel), 3Al2O3•2SiO2 (mullite, which is located along the right-side border of Figure 18-2), and Al2TiO5 or Al2O3•TiO2 (aluminum titanate). The spinel structure is used in a glass-infiltrated ceramic (In-Ceram Spinell) for applications in which greater translucency is required. Most nonoxide ceramics are not of practical use in dentistry either because of their high processing temperatures, complex processing methods, or their unesthetic color and opacity. Such ceramics include borides (TiB2, ZrB2), carbides (B4C, SiC, TiC, WC), nitrides (AIN, BN, Si3N4, TiN), selenide (ZnSe), silicide (MoSi2), sialon (Si3N4 with Al2O3), and syalon (Si3N4 with Al2O3 and Y2O3).
History of Dental Ceramics
Because natural minerals are not tooth-colored, subsequent civilizations used a variety of materials to produce simulated teeth. In approximately 700 B.C., the Etruscans made artificial teeth of ivory and bone, human teeth, and animal teeth (possibly oxen) that were held in place by gold wires or flat bands and rivets (Figure 18-3). Animal bone and ivory from hippopotami and elephants were used for many years thereafter.
Human teeth that were sold by the poor and teeth obtained from the dead were also used for centuries thereafter, but dentists generally avoided this option. One of the first sets of dentures made for U.S. President George Washington contained extracted teeth (Figure 18-4, center) but later his dentures were made of hippopotamus ivory (Figure 18-4, left). The ivory tooth forms were supported in the maxillary denture by a gold palatal plate and the dentures were retained by pressure applied by coiled springs attached to the sides of the denture bases. President Washington was inaugurated in 1789 with one remaining tooth. He suffered from poor oral health (although it is reported that he had brushed his teeth regularly with tooth powder). His poor-fitting dentures caused him much discomfort during his presidency (1789−1797) and until his death, in 1799, at the age of 67. None of his dentures were ever made of wood, contrary to erroneous reports circulated since his death.


B, Portrait of President Washington, who died on December 14, 1799. Technology for producing porcelain denture teeth was not available until 1825, although these prostheses were not refined until vulcanized rubber denture bases were developed in 1839.
Two of the most important breakthroughs responsible for the long-standing superb esthetic performance and clinical survival probabilities of metal-ceramic restorations are described in the patents of Weinstein and Weinstein (1962) and Weinstein et al. (1962). One of these patents identified the formulations of feldspathic porcelain that enabled the systematic control of the sintering temperature and coefficient of thermal expansion. The other patent described the components that could be used to produce alloys that bond chemically to and that are thermally compatible with the feldspathic porcelains. The first commercial porcelain was developed by VITA Zahnfabrik in about 1963. Although the first VITA porcelain products were known for their esthetic properties, the subsequent introduction of the more versatile Ceramco porcelain led to thermal expansion behavior that allowed this porcelain to be used safely with a wider variety of alloys.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses