16 Nonodontogenic Intraosseous Lesions
Tori and Exostoses
Clinical Findings
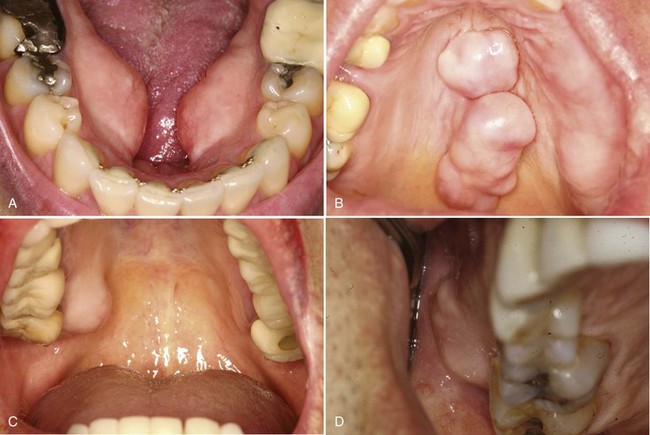
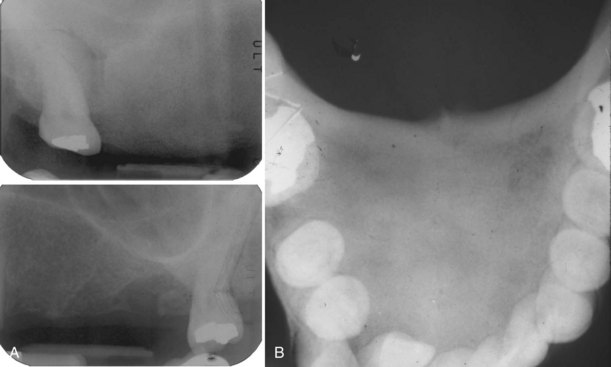
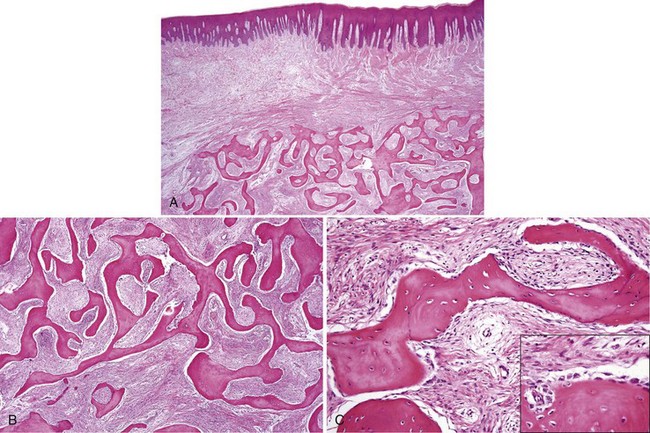
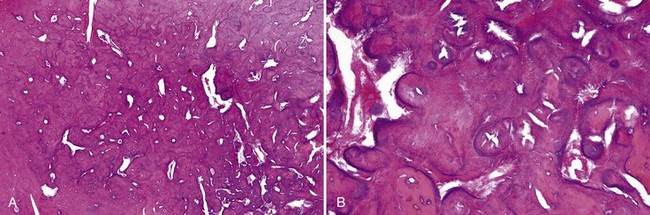
• Tori: 2% to 10% of adults; exophytic, hard, uninodular or multinodular bony masses covered by mucosa that may be ulcerated from trauma; torus palatinus and torus mandibularis on the midline of palate and lingual mandible (usually bilateral and symmetric), respectively (Fig. 16-1, A and B); often site of bisphosphonate-associated osteonecrosis
• Exostoses: 27% of adults; male predilection (5 : 1); outgrowths of bone, nodular or sessile frequently on buccal aspects of mandible and maxilla or ascending arch of the palate and more than 90% having concurrent tori (see Fig. 16-1, C and D); exostoses possibly show rapid growth in some patients
Etiopathogenesis and Histopathologic Features
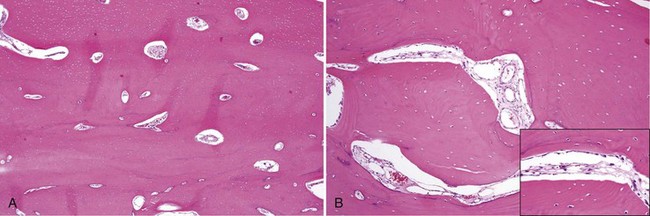
• Dense lamellar bone with well-spaced small osteocytes, fibrovascular tissue in haversian canals, variable osteoblastic rimming, especially if inflamed (Fig. 16-2); fatty marrow often present
• Long, sweeping collagen bundles (lamellae) noted under polarized light (Fig. 16-3)
Differential Diagnosis
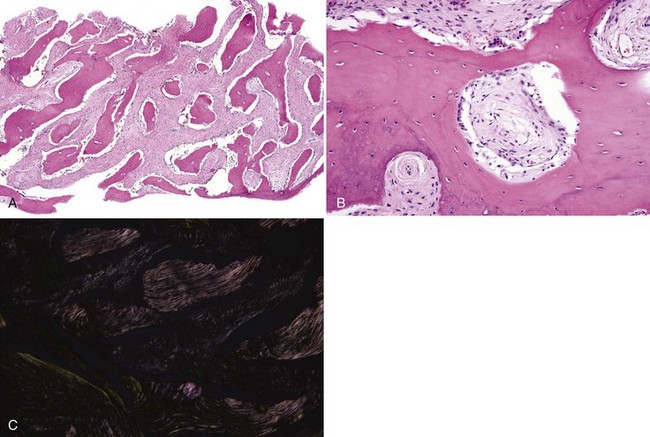
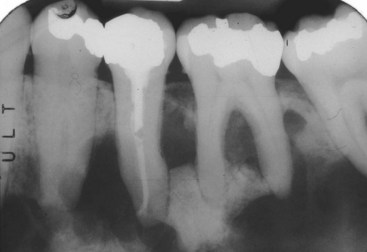
• Dense bone island, bone scar, or idiopathic osteosclerosis is not exophytic and is an asymptomatic intraosseous radiopacity not related to teeth that is discovered on routine radiography (Fig. 16-4, A); biopsy is not necessary unless the lesion is radiographically progressive; histopathology is similar and bone is often sclerotic.
• Condensing osteitis is similar to idiopathic osteosclerosis but is found at or very close to the apices of teeth and is likely reactive to chronic occlusal trauma or low-grade inflammation or odontogenic infection; biopsy is not necessary (see Fig. 16-4, B).
• True osteomas are associated with Gardner syndrome (autosomal dominant condition associated with mutation in the APC gene and development of colonic polyps and carcinoma, desmoid tumors, supernumerary teeth, and skin cysts).
Chohayeb AA, Volpe AR. Occurrence of torus palatinus and mandibularis among women of different ethnic groups. Am J Dent. 2001;14:278-280.
Haugen LK. Palatine and mandibular tori. A morphologic study in the current Norwegian population. Acta Odontol Scand. 1992;50:65-77.
Jainkittivong A, Langlais RP. Buccal and palatal exostoses: prevalence and concurrence with tori. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:48-53.
Seah YH. Torus palatinus and torus mandibularis: a review of the literature. Aust Dent J. 1995;40:318-321.
White S, Pharoah M. Oral Radiology: Principles and Interpretation, 6th ed. St. Louis: Mosby; 2009.
Yonetsu K, Yuasa K, Kanda S. Idiopathic osteosclerosis of the jaws: panoramic radiographic and computed tomographic findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:517-521.
Fibro-Osseous Lesions
Clinical Findings
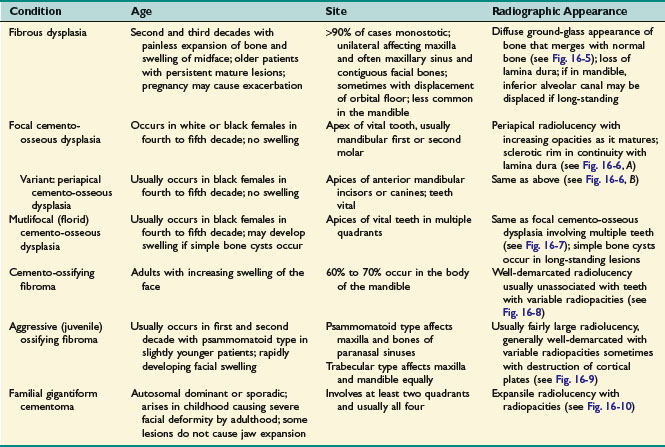
• Please see Table 16-1 and Figs. 16-5 to 16-10.
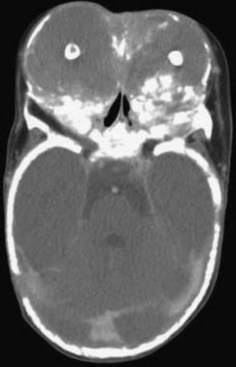
FIGURE 16-9 Aggressive ossifying fibroma: huge radiolucent and radiopaque lesion of the maxilla.
(Courtesy of Dr. Bonnie Padwa, Harvard School of Dental Medicine, Boston, Mass.)
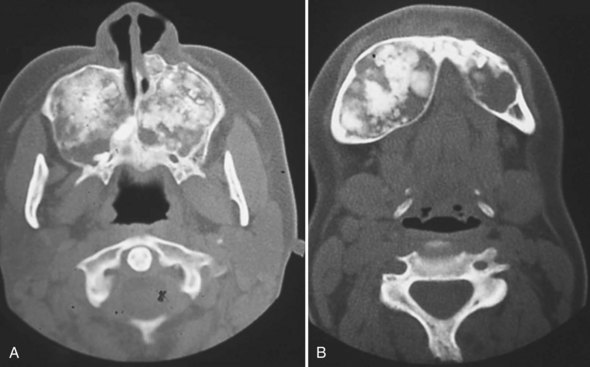
FIGURE 16-10 Familial gigantiform cementomas: marked expansion of maxilla (A) and mandible (B) by radiolucent-radiopaque process.
(From Abdelsayed RA, Eversole LR, Singh BS, Scarbrough FE. Gigantiform cementoma: clinicopathologic presentation of 3 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:438-444.)
Etiopathogenesis and Histopathologic Features
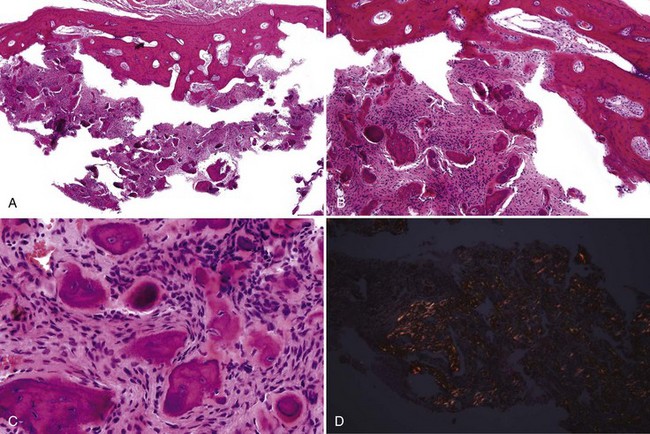
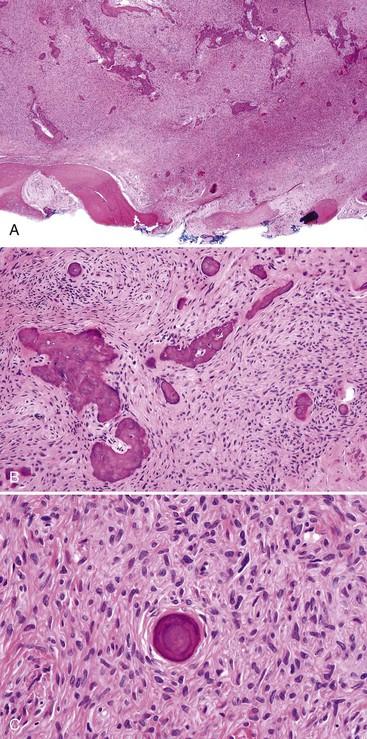
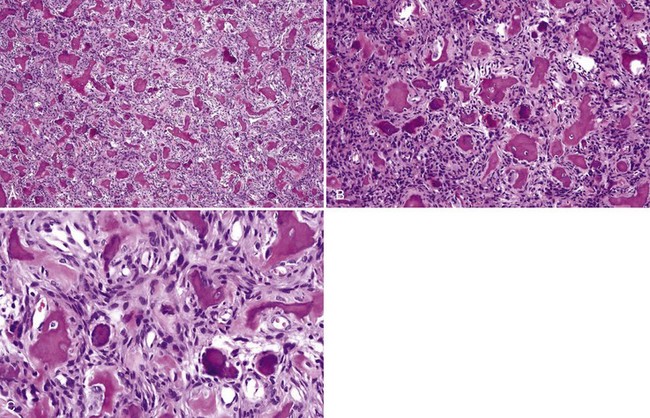
• Fibrous dysplasia consists of a proliferation of benign fibrous tissue and either small, delicate, or curvilinear trabeculae of woven bone in early lesions, or thicker and denser trabeculae of woven or lamellar bone in mature lesions; osteoblastic rimming and occasional osteoclasts may be seen focally, especially in early and inflamed lesions; there is variable vascularity (Figs. 16-11 and 16-12); aneurysmal bone cyst–like areas may be seen.
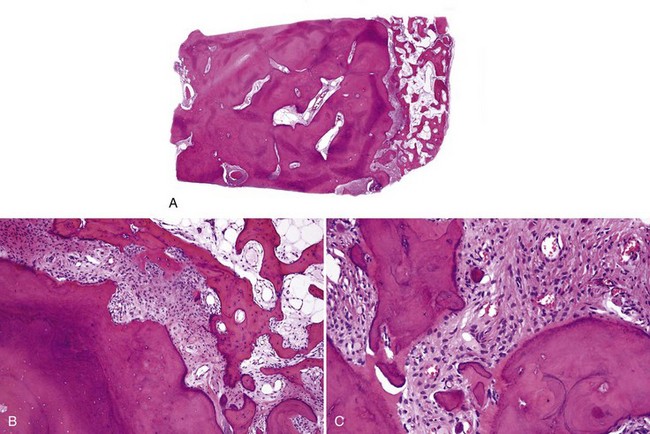
• Cemento-osseous dysplasia consists of a cellular proliferation of spindled benign fibroblast-like cells and deposition of osteoid, woven bone, cementum droplets, or a mixture thereof; mature lesions consist of a mass of cementum that has a rounded, globular appearance with few cementocytes (sclerotic cemental mass) (Figs. 16-13 to 16-15); cells that rim bone and cementum are often osteoblast-like.
• Cemento-ossifying fibroma consists of cellular proliferation of spindled fibroblast-like cells that deposit osteoid, trabeculae of woven bone, and cementum droplets in varying proportions; osteoblastic rimming is common (Fig. 16-16).
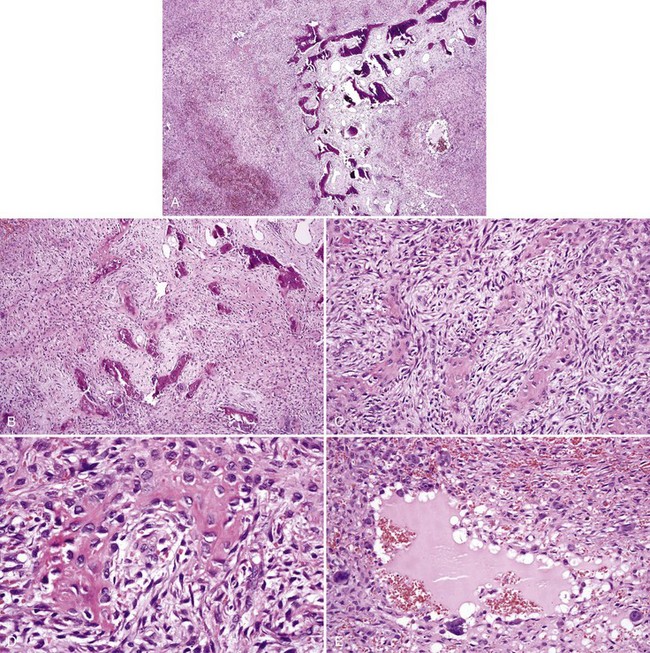
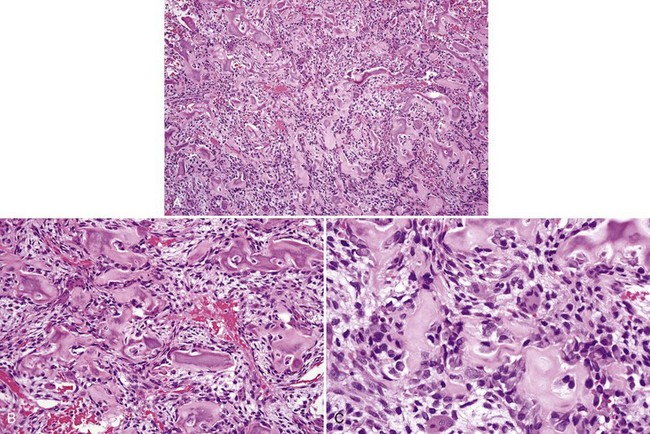
• Aggressive ossifying fibroma (desmo-osteoblastoma) consists of a cellular proliferation of plump and stellate fibroblast-like cells with large, slightly atypical nuclei, clusters of multinucleated giant cells, and fresh hemorrhage; trabecular variant has abundant woven bone surrounding large osteocytes with many osteoblasts (Figs. 16-17 and 16-18); psammommatoid variant has many basophilic cementum droplets or psammomatoid bodies, most of which are of uniform size (Fig. 16-19); this subtype appears similar to familial gigantiform cementoma.
• Familial gigantiform cementoma is similar to ossifying fibroma, especially psammomatoid type.
Differential Diagnosis
• Cementoblastoma shows a proliferation of cementoblasts (resembling osteoblasts) with radiating seams of cementum (see Chapter 15).
• Osteoblastoma shows a proliferation of osteoblasts composed of large cells with eccentric nuclei, often multilayered against the bone, osteoclasts on the same trabeculae, and prominent ectatic vessels (Fig. 16-20); bone is basoophilic, sometimes with many resting and reversal lines.
• Renal osteodystrophy from secondary hyperparathyroidism may manifest with similar histology to fibrous dysplasia; clinical features of renal failure with hypercalcemia differentiate the two.
Management and Prognosis
• Fibrous dysplasia: recontouring is for aesthetics only.
• Cemento-osseous dysplasia: no treatment necessary; however, sclerotic masses of cementum are hypovascular and extractions may lead to osteomyelitis; simple bone cysts are associated with long-standing lesions (Fig. 16-21).
• Cemento-ossifying fibroma: curettage, enucleation, or excision is curative.
• Aggressive ossifying fibroma: wide excision is the treatment of choice; recurrence is 30% to 50%, primarily because of incomplete excision.
Abdelsayed RA, Eversole LR, Singh BS, Scarbrough FE. Gigantiform cementoma: clinicopathologic presentation of 3 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:438-444.
Alawi F. Benign fibro-osseous diseases of the maxillofacial bones. A review and differential diagnosis. Am J Clin Pathol. 2002;118(suppl):S50-S70.
Damm DD, Neville BW, McKenna S, et al. Macrognathia of renal osteodystrophy in dialysis patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:489-495.
El-Mofty S. Psammomatoid and trabecular juvenile ossifying fibroma of the craniofacial skeleton: two distinct clinicopathologic entities. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:296-304.
Eversole LR, Leider AS, Nelson K. Ossifying fibroma: a clinicopathologic study of sixty-four cases. Oral Surg Oral Med Oral Pathol. 1985;60:505-511.
Eversole R, Su L, ElMofty S. Benign fibro-osseous lesions of the craniofacial complex. A review. Head Neck Pathol. 2008;2:177-202.
MacDonald-Jankowski D. Fibrous dysplasia: a systematic review. Dentomaxillofac Radiol. 2009;38:196-215.
Macdonald-Jankowski DS, Li TK. Fibrous dysplasia in a Hong Kong community: the clinical and radiological features and outcomes of treatment. Dentomaxillofac Radiol. 2009;38:63-72.
Rossbach HC, Letson D, Lacson A, et al. Familial gigantiform cementoma with brittle bone disease, pathologic fractures, and osteosarcoma: a possible explanation of an ancient mystery. Pediatr Blood Cancer. 2005;44:390-396.
Su L, Weathers DR, Waldron CA. Distinguishing features of focal cemento-osseous dysplasias and cemento-ossifying fibromas. I. A pathologic spectrum of 316 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:301-309.
Su L, Weathers DR, Waldron CA. Distinguishing features of focal cemento-osseous dysplasia and cemento-ossifying fibromas. II. A clinical and radiologic spectrum of 316 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:540-549.
Summerlin DJ, Tomich CE. Focal cemento-osseous dysplasia: a clinicopathologic study of 221 cases. Oral Surg Oral Med Oral Pathol. 1994;78:611-620.
Young SK, Markowitz NR, Sullivan S, et al. Familial gigantiform cementoma: classification and presentation of a large pedigree. Oral Surg Oral Med Oral Pathol. 1989;68:740-747.
Paget Disease of Bone (Osteitis Deformans)
Clinical Findings
• Affects 1% to 2% of the white population (usually men) older than age 55, with highest frequency in the United Kingdom and lowest frequency in Asia; familial history in 15% to 40% of cases; first sign may be bone pain and headaches; deposition of bone around skull foramina may lead to neurologic symptoms, such as hearing or visual loss; poor fit of dentures or hats and spacing between teeth may occur from expansion of skull and jaw bones.
• Radiographs in early stages show osteoporotic changes; in the mixed stage, there is thickening of bones with mixed radiolucency and radiopacity (“cotton wool appearance”) with bone sclerosis being more marked as disease progresses (Fig. 16-22).
• High levels of bone-specific alkaline phosphatase and urine hydroxyproline are noted.
Etiopathogenesis and Histopathologic Findings
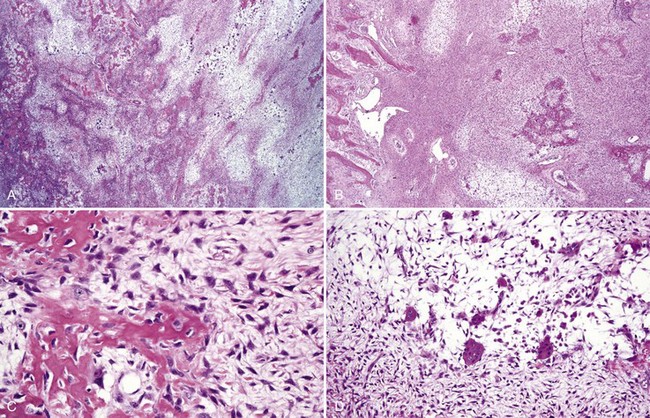
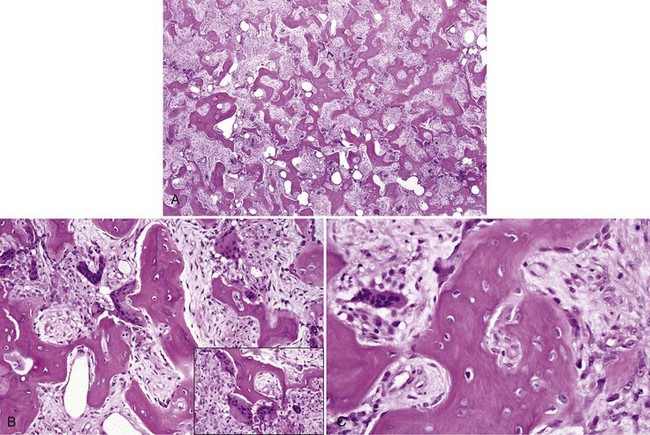
• The haphazard deposition of bone results in a mosaic pattern and many resting and reversal lines; numerous giant, irregular-shaped, hypernucleated osteoclasts are present; many osteoblasts are noted on surface of bone trabeculae, and there is marked hypervascularity in the mixed stage (Fig. 16-23).
• Giant cell tumors occur infrequently in association with this disease.
• Microtubular inclusions are seen on ultrastructural studies.
Bullough P. Orthopaedic Pathology, 5th ed. St. Louis: Mosby; 2010.
Hansen MF, Seton M, Merchant A. Osteosarcoma in Paget’s disease of bone. J Bone Miner Res. 2006;21(suppl 2):P58-P63.
Hoch B, Hermann G, Klein MJ, et al. Giant cell tumor complicating Paget disease of long bone. Skeletal Radiol. 2007;36:973-978.
Morissette J, Laurin N, Brown JP. Sequestosome 1: mutation frequencies, haplotypes, and phenotypes in familial Paget’s disease of bone. J Bone Miner Res. 2006;21(suppl 2):P38-P44.
Ralston SH, Langston AL, Reid IR. Pathogenesis and management of Paget’s disease of bone. Lancet. 2008;372:155-163.
Roodman GD. Insights into the pathogenesis of Paget’s disease. Ann N Y Acad Sci. 2010;1192:176-180.
Seitz S, Priemel M, Zustin J, et al. Paget’s disease of bone: histologic analysis of 754 patients. J Bone Miner Res. 2009;24:62-69.
Central Giant Cell Granuloma (Aggressive and Nonaggressive Giant Cell Lesion, Central Giant Cell Lesion, Central Giant Cell Reparative Granuloma)
Clinical Findings
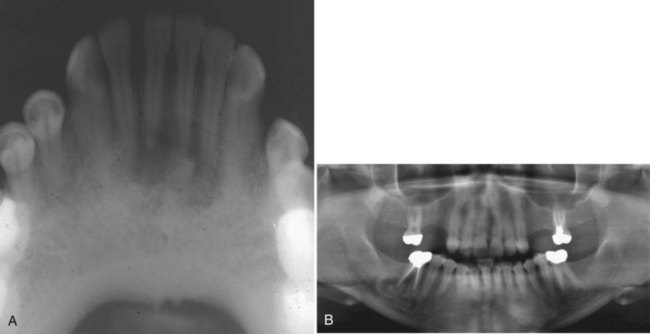
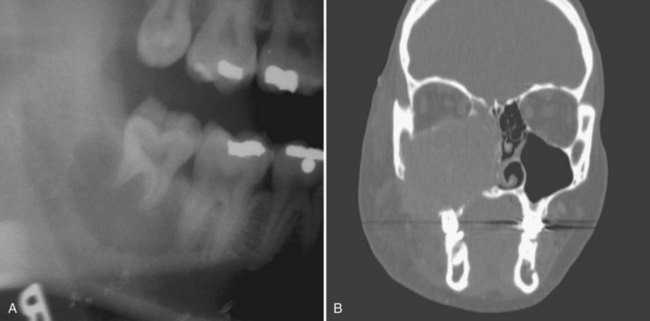
• Lesions occur in the first to third decade of life, often presenting as a unilocular or multilocular radiolucency of the mandible (75% of cases), usually in the anterior or body (Fig. 16-24, A).
• Aggressive type is less than 5 cm, recurs (primary criteria) or shows at least three of the following secondary criteria: rapid growth, root resorption, tooth displacement and thinning, or perforation of the cortical plate (see Fig. 16-24, B).
• Multiple giant cell lesions are often seen in Noonan-like multiple giant cell lesion syndrome (associated with PTPN11 and SOS1 mutations, among others) and in hyperparathyroidism.
Etiopathogenesis and Histopathologic Features
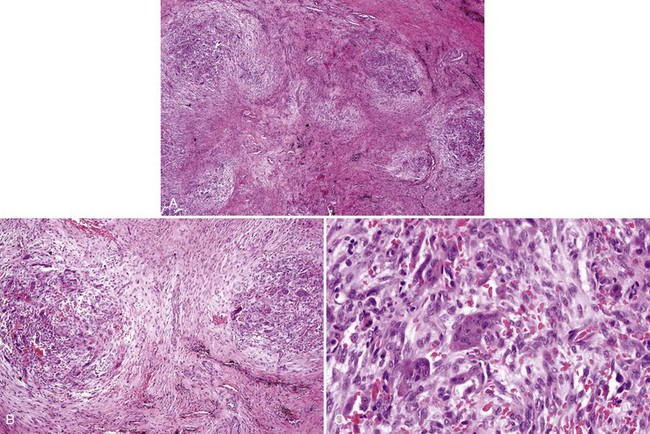
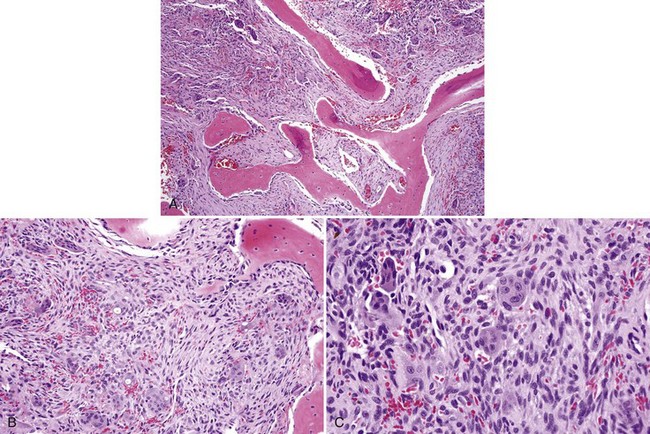
• Proliferation of mononuclear cells and clusters of multinucleated osteoclast-like giant cells with abundant fresh hemorrhage, siderophages, and reactive osteoid and woven bone formation; giant cells have 20 to 30 nuclei; mononuclear cells often show mitotic activity (Figs. 16-25 and 16-26).
• More extensive vasculature may correlate with aggressive lesions.
• Most giant cells and approximately 10% of mononuclear cells stain for tartrate-resistant acid phosphatase, CD68, cathepsin, and MMP-9; in situ hybridization studies show mRNA for osteoprotegrin within giant cells and RANK and RANKL within stromal cells; both show variable staining for glucocorticoid and calcitonin receptors.
Differential Diagnosis
• Brown tumor of hyperparathyroidism is indistinguishable from central giant cell granuloma; high parathormone levels and serum calcium differentiate the two conditions.
• Giant cell tumors of bone tend to have sheets rather than clusters of giant cells, which contain more nuclei, although distinction may be difficult; differences in histopathology may predict more aggressive behavior.
• Aneurysmal bone cyst consists of large blood-filled spaces lined by giant cells.
• Cherubism is distinguished by its typical bilateral presentation.
• Abundant woven bone deposition may suggest a fibro-osseous lesion, but giant cells usually are not a feature in the latter.
Management and Prognosis
• Recurrence in 10% to 70% of cases depending on mode of therapy.
• Curettage, excision, resection, intralesional steroid injections, calcitonin nasal spray; enucleation and/or tumor debulking with adjuvant interferon-α therapy have been successful in resolving lesions completely (Fig. 16-27).
• Other treatment modalities are bisphosphonate and imatinib therapy.
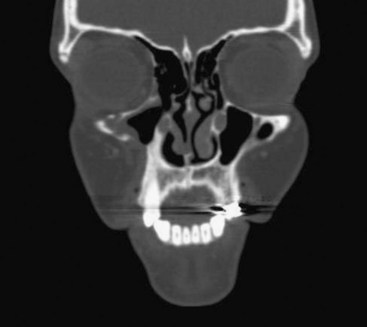
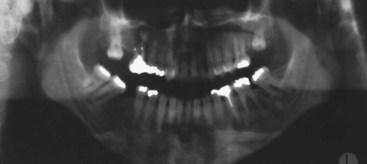
FIGURE 16-27 Aggressive central giant cell granuloma (same patient as in Figure16-24, B): coronal CT exhibits resolution of right maxillary lesion 3 years after enucleation and 2 years after completion of treatment with systemic interferon therapy.
(Courtesy of Dr. Leonard B. Kaban and Dr. Maria Troulis, Harvard School of Dental Medicine and Massachusetts General Hospital, Boston, Mass.)
Auclair PL, Cuenin P, Kratochvil FJ, et al. A clinical and histomorphologic comparison of the central giant cell granuloma and the giant cell tumor. Oral Surg Oral Med Oral Pathol. 1988;66:197-208.
Chuong R, Kaban LB, Kozakewich H, Perez-Atayde A. Central giant cell lesions of the jaws: a clinicopathologic study. J Oral Maxillofac Surg. 1986;44:708-713.
de Lange J, van Rijn RR, van den Burg H, et al. Regression of central giant cell granuloma by a combination of interferon and imatinib: a case report. Br J Oral Maxillofac Surg. 2009;47:59-61.
Dewsnup NC, Susarla SM, Abulikemu M, et al. Immunohistochemical evaluation of giant cell tumors of the jaws using CD34 density analysis. J Oral Maxillofac Surg. 2008;66:928-933.
Gebre-Medhin S, Broberg K, Jonson T, et al. Telomeric associations correlate with telomere length reduction and clonal chromosome aberrations in giant cell tumor of bone. Cytogenet Genome Res. 2009;124:121-127.
Gleason BC, Kleinman PK, Debelenko LV, et al. Novel karyotypes in giant cell-rich lesions of bone. Am J Surg Pathol. 2007;31:926-932.
Kaban LB, Troulis MJ, Wilkinson MS, et al. Adjuvant antiangiogenic therapy for giant cell tumors of the jaws. J Oral Maxillofac Surg. 2007;65:2018-2024. discussion 2024
Liu B, Yu SF, Li TJ. Multinucleated giant cells in various forms of giant cell containing lesions of the jaws express features of osteoclasts. J Oral Pathol Med. 2003;32:367-375.
Resnick CM, Margolis J, Susarla SM, et al. Maxillofacial and axial/appendicular giant cell lesions: unique tumors or variants of the same disease? A comparison of phenotypic, clinical, and radiographic characteristics. J Oral Maxillofac Surg. 2010;68:130-137.
Tobon-Arroyave SI, Franco-Gonzalez LM, Isaza-Guzman DM, et al. Immunohistochemical expression of RANK, GRalpha and CTR in central giant cell granuloma of the jaws. Oral Oncol. 2005;41:480-488.
Vered M, Buchner A, Dayan D. Immunohistochemical expression of glucocorticoid and calcitonin receptors as a tool for selecting therapeutic approach in central giant cell granuloma of the jawbones. Int J Oral Maxillofac Surg. 2006;35:756-760.
Whitaker SB, Waldron CA. Central giant cell lesions of the jaws. A clinical, radiologic and histopathologic study. Oral Surg Oral Med Oral Pathol. 1993;75:199-208.
Cherubism
Clinical Findings
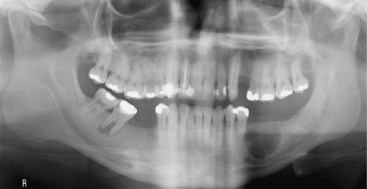
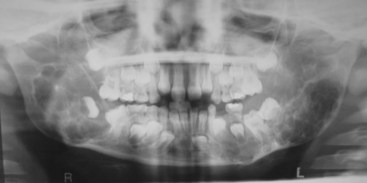
• Condition occurs most frequently in first decade; bilateral multiloculated radiolucencies of the posterior mandible and ascending ramus often result in tooth displacement, delayed tooth eruption, and loosening of teeth (Fig. 16-28); involvement of the maxilla causes eyes to turn upward, resembling a cherub; a unilateral lesion may be the first presentation.
• Lesions cause bony expansion and swelling up to age 12; symptoms are usually quiescent for a few years and then regress over many years beginning in adolescence.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses