16
Healing of Chronic Apical Periodontitis
Introduction
Modern maxillofacial radiology has several advanced methodologies for diagnostic assessment of endodontically related diseases and conditions. Some of these are used mainly in hospitals and universities; others are gradually being employed by expansive public and private clinics. Cone beam computed tomography (CBCT) has become extremely useful in selected endodontic cases. However, cost and, particularly, radiation concerns limit the general applicability of this and other relatively advanced methods.
The periapical radiograph therefore remains the primary source of diagnostic insight beyond clinical examination in endodontic practice. It has a history of approximately one century, during which time the whole dental profession has accumulated and shared information derived from its application, especially information related to endodontics. In fact, the concepts that have developed regarding the three-dimensional features of apical periodontitis are based and rely on the two-dimensional renderings of the periapical radiograph.
Aspects of cost and radiation concern also dictate that for large-scale epidemiological studies and in clinical research, the periapical radiograph (or the orthopanthomogram) often is the only method available that can give sufficiently precise data.
So while the amount of information that is potentially available through the use of CBCT, it is imperative that we systematize and maximize the information that can be obtained with the conventional, periapical radiograph.
Radiographic Features of Apical Periodontitis
Chronic Apical Periodontitis
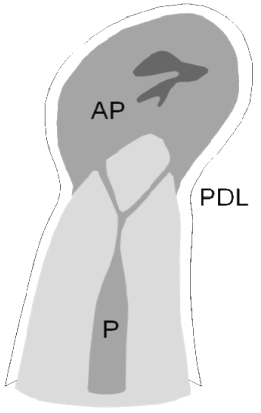
Periodontitis resulting from pulp infection usually locates to the area of the apical entrance of vessels and nerves to the pulp, hence the conventional term apical periodontitis. Periodontal inflammation from pulp infection may also occur in lateral and furcal infection, with optional nomenclatures such as lateral, furcal, and periradicular periodontitis. The pathognomonic, radiographic features are the same: the apical lesion has a droplet-shaped, radiolucent appearance, the periodontal ligament (PDL) tapers off into the outer periphery of the lesion, and the lamina dura is absent at the apex and/or other portals of pulp entry. The size of the lesion varies from but a few millimeters to several centimeters (Figure 16.1).
Figure 16.1 Established, apical periodontitis. The radiolucent lesion has a droplet-shaped appearance; the PDL tapers off toward the outer periphery of the lesion; and the lamina dura is absent at the apex. The lesion itself starts from the infected contents of the root canal
(Reprinted from Ørstavik and Larheim, 2008, with permission from John Wiley & Sons).
Incipient and Acute Apical Periodontitis
These may show minimal or no changes detectable in radiographs. In humans, little is known about the speed and dynamics of the initial changes in bone mineral content and structural changes occurring in the initial stages of infection. In some cases, a diffuse reduction in mineral content may be detectable; in others, incipient or low-grade infection may be associated with structural disorganization of bone in the periapical area (Brynolf, 1967) (Figure 16.2).
Figure 16.2 Incipient or low-grade infection may be associated with structural disorganization of bone in the periapical area. (A) Symptomatic with acute apical periodontitis. (B) 2 weeks later the lesion is entering a chronic phase. (C) Control after 1 year.
More is known from animal studies. Within a period of weeks to several months prior to the establishment of a periapical granuloma or cyst, tissue changes occur which may be detectable as a reorganization of the area to become occupied by the lesion (Friedman et al., 1997; Ørstavik & Larheim, 2008).
Periapical Cyst
Periapical cysts are traditionally divided into two categories: the bay or pocket cyst and the “true” periapical cyst (Nair et al., 1996; Simon, 1980). The lumen of the former is in continuity with the root canal lumen; the “true” cyst is dissociated from the root and may therefore be resistant to conventional root canal treatment (RCT) and need surgical extirpation. It is debatable whether the radiographic appearance holds features helpful in differentiating between a cyst and a granuloma (Shrout et al., 1993; White et al., 1994). The traditional belief that a radiopaque rim is indicative of a cyst has been challenged (Ricucci et al., 2006). What is more generally agreed is that with increasing size, possibly also age, of the lesion, the greater the likelihood of finding cystic elements in the lesion (Carrillo et al., 2008; Kizil and Energin, 1990).
Tissue Responses to Materials and Procedures
Responses to Foreign Objects and Endodontic Filling Materials
While extruded root filling material may have a negative prognostic influence on healing in the treatment of chronic apical periodontitis (Sjögren et al., 1990), the materials themselves seem to have little influence on bone mineral content or structure. It is traditionally accepted, though, that a small radiolucent zone may persist around such surplus material at the root canal orifice (Strindberg, 1956). With some materials, such as resins and mineral trioxide aggregate, one may hope for better tissue integration with no or smaller radiolucent zone peripheral to the material (Rud et al., 1991; Torabinejad et al., 1995).
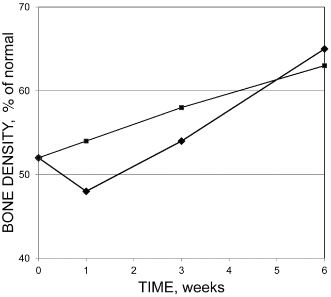
Treatment and filling procedures may cause a transient loss of mineral (increased radiolucency) at the periapex (Benfica e Silva et al., 2010; Ørstavik, 1991), but this is reversible and does not normally initiate or sustain either acute or chronic apical periodontitis (Sjögren et al., 1990) (Figure 16.3). However, extruded material has been associated with granuloma and cyst formation (Koppang et al., 1989; Love and Firth, 2009).
Figure 16.3 Average change in bone density (ratio of diseased area vs. normal peripheral bone in percent) after treatment of apical periodontitis with Sealapex (squares) or ProcoSol (diamonds)
(Modified from Ørstavik, 1991, with permission from John Wiley & Sons).
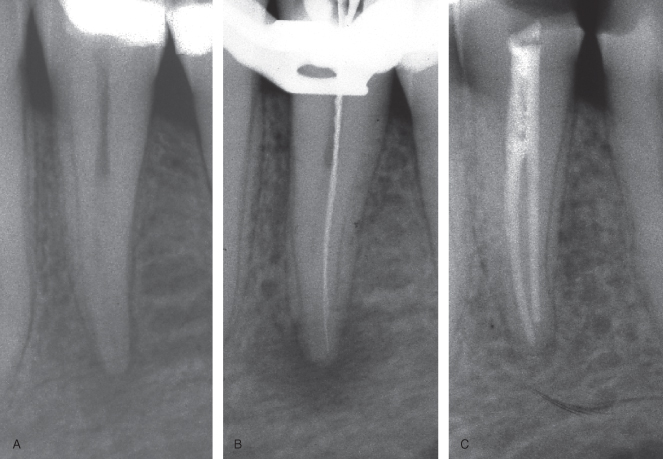
Responses to Surgical Procedures
Radiographic analyses of jaw bones after apical surgery present particular problems. The cavity created during surgery becomes the starting point for follow-up controls of healing. Two processes may now be operating: on the one hand, the blood clot will become organized and start to mineralize. On the other, residual infection may in part or totally interfere with the healing of the surgical site. The ensuing radiographic image may be difficult to interpret, and special caution must be exercised when assessing the healing (Figure 16.4).
Figure 16.4 Ambiguity in interpretation of healing following surgical endodontics. The 1-year follow-up radiograph (C) shows clear sign of healing, but residual infection of the first premolar cannot be ruled out. (A) Case on admission. (B) Immediate postoperative radiograph.
Thus, in a classic study comparing the radiographic healing of chronic apical periodontitis treated conventionally or surgically, it was found that short-term observation periods (6 months) tended to favor surgical treatment, whereas the results were considered just as good or better for conventional retreatment after 1–2 years of observation (Kvist and Reit, 1999).
Characteristics of Healing of Chronic Apical Periodontitis
Dynamics of the Healing Process and Its Reflection in Radiographic Changes
The biological processes leading to the clearing of an apical granuloma or cystic lesion are poorly understood. The fibrous character of most lesions makes it likely that healing requires a substantial amount of time in all cases. Furthermore, factors related to lesion size and location, the patient’s general health and constitution, and residual infection in the area, (Brummer and van Wyk 1987, Fouad and Burleson, 2003; Segura-Egea et al., 2005) may contribute to variations in the healing pattern. While it may be tempting to look at bony healing as a balloon which shrinks in size, mineralization may also start irregularly from within the lesion, and as spicules penetrating from the periphery toward the central area. Such different patterns of healing are likely to produce different radiographic appearances. For observation of so-called “complete” healing, individual cases may have to be followed for several years, with “late” healing observed as much as 17–27 years postoperatively (Molven et al., 2002) (Figure 16.5).
Figure 16.5 For observation of so-called “complete” healing, individual cases may have to be followed for several years, with “late” healing observed decades after treatment. (a) Lesion at 17 years after treatment; (b) healing after 27 years
(Reprinted from Molven et al., 2002, with permission from John Wiley & Sons).
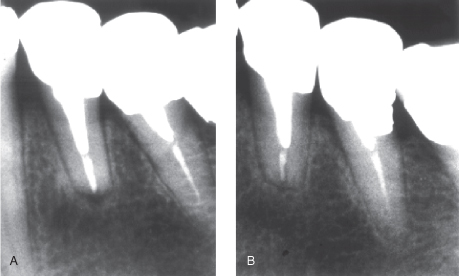
Time Course of Healing Processes
Computer-assisted means of radiographic analysis have indicated that in many, if not most cases, increased radiographic density may often be seen after a few weeks and quite regularly at 3–6 months (Kerosuo and Ørstavik, 1997). Also, by conventional radiographic analyses, changes may be detected early (Figure 16.6).
Figure 16.6 In many, if not most cases, signs of healing may often be seen after a few weeks and quite regularly at 12–26 weeks
(Modified from Trope et al., 1999, with permission from Elsevier Ltd.).
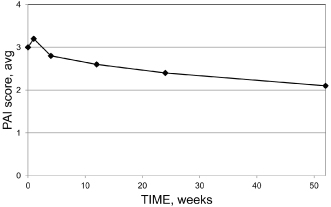
However, a period of 1 year may be necessary to assess the overall outcome after treatment of chronic apical periodontitis; even those cases that require longer time for complete healing generally improve sufficiently to be classified as clinically successful after 1 year (Cvek, 1972; Ørstavik, 1996; Reit, 1987) (Figure 16.7).
Figure 16.7 Cumulative percentage of teeth that will eventually heal at yearly intervals from endodontic treatment of teeth with apical periodontitis
(Modified from Ørstavik, 1996, with permission from John Wiley & Sons: Solid squares, data from Cvek et al. (1976); solid diamonds, data from Ørstavik et al., 1987).
Assessment of Healing in Clinical Practice
The radiographic control of healing is usually done by comparing a recall radiograph with one taken at the time of treatment. Teaching and clinical practices vary with regard to the criteria used to make a diagnosis of posttreatment disease (Friedman, 2008). Computer-assisted, automated means of analyzing and comparing periapical radiographs are available, but documentation of precision and accuracy is weak. Methods for determination of healing of apical periodontitis include ratio measurements of bone density in the lesion versus normal bone surrounding it; digital subtraction of densities in corresponding areas in two radiographs; and measurement of the lesion size (see below).
Clinical Experiments and Epidemiology
Prevention and Treatment
The fundamental difference between RCT of infected and noninfected teeth should be recognized. The prognosis of RCT after vital pulp extirpation is clearly superior to that of RCT on infected teeth with chronic apical periodontitis (Kerekes and Tronstad, 1979; Ng et al., 2011; Ørstavik et al., 1987). With the application of more sensitive means of detection (CBCT), it is likely that even more teeth may show residual infection than has currently been assumed (de Paula-Silva et al., 2009). It is most unfortunate that clinical and experimental studies are still carried out where the two preoperative diagnoses are mixed in the design. The situation is more or less unavoidable in epidemiology, which makes the interpretation of such data very complex (see below).
Treatment of Teeth with No Preoperative Lesion
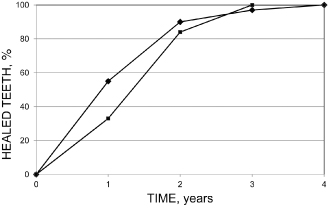
The development of a granuloma is usually asymptomatic, and primary chronic apical periodontitis (CAP) is often detected in a radiograph taken for other reasons. A time course for its development in humans is hard to establish for primary apical periodontitis. However, after root filling of vital teeth, many studies have monitored the outcome of treatment by repeated and regular radiographic follow-ups. It has been found that some 90% of teeth that develop a lesion (secondary apical periodontitis) can be detected with conventional radiographs after 1 year (Ørstavik, 1996) (Figure 16.8).
Figure 16.8 Some 90% of teeth that develop a lesion (secondary apical periodontitis) can be detected with conventional radiographs after 1 year. Bars are standard deviations
(Reprinted from Ørstavik, 1996, with permission from John Wiley & Sons).
The Success/Failure Concept in Everyday Practice and Clinical Research
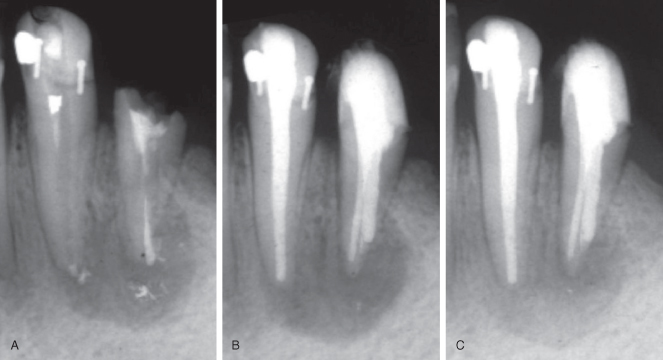
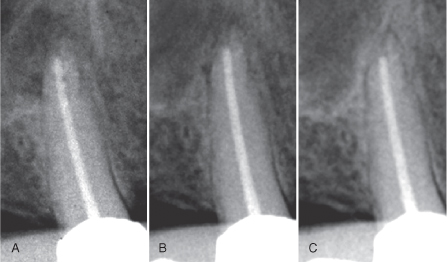
Endodontic success is usually described as the absence, clinically and radiographically, of signs of apical periodontitis. In practice, the radiographic analysis is carried out by comparison of recall radiographs with preoperative or immediate postoperative radiographs of the tooth in question. For teeth without a preoperative lesion, a failure is recorded when the periapical area becomes more radiolucent; otherwise, it is a success. For teeth with a lesion, the comparison looks for healing, which may be recorded when the change is clearly in favor of the recall X-ray (Figure 16.9).
Figure 16.9 Conventional monitoring of healing of apical periodontitis. Compared with the immediate postoperative radiograph (A), bone is forming after 3 months (B), and complete healing is observed after 12 months (C).
Otherwise it is a failure. With this scoring method, success rates are generally recorded as very high for both diagnostic categories. In the individual case, the assessment of success/failure is further blended with the patient’s and operator’s predefined goal of the procedure (Friedman, 2008). The problems with such assessment in scientific studies are lack of agreement on what constitutes “appearance of a lesion” for vital tooth treatment, and when an increase in bone density at the apex of a tooth with a preoperative lesion is large enough to be termed “normal,” “healing,” or “healed.” It is difficult/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


