Chapter 15
Headaches with a focus on chronic daily headache medications
15.1 Introduction to Headaches
The International Headache Society (IHS) separates headaches into two forms: primary (no underlying etiology) and secondary (where an underlying cause is present).1 Primary headaches can be further divided into those that are episodic and those that are continuous or quite frequent. This chapter discusses both, although our focus is clearly on primary continuous headaches, and it is divided into five sections. First we briefly discuss some of the acute but potentially very dangerous secondary headaches followed by a discussion of the features, etiology, and common methods of treatment of the two most frequently seen primary episodic headaches (tension-type and migraine) that plague patients. The last part of this section covers some of the other, rarer episodic headaches. In Sections 15.2 and 15.3 we discuss the various etiologies and mechanisms that are thought to contribute to headache causation as well as treatment methods for the episodic headaches. In Section 15.4, we then review the group of disorders described as chronic daily headaches. We will discuss how migraines and tension-type headaches can transform from an episodic form of the disease into a chronic form over time and we examine in detail the factors that make this happen. Finally, and most important, in Sections 15.4 and 15.5, we describe the common chronic daily headaches and how to manage them. Unfortunately, headache patients may present with more than one type of headache, confusing the picture for the clinician.
15.1.A Dangerous (Secondary) Headaches (Diagnosis)
Secondary headaches are caused by a specific structural or medical condition and are often life-threatening. Fortunately, they are rare and account for about 1% of all headaches in primary-care settings.2 In the revised classification put forth by the International Headache Society (IHS-2), they are subdivided into eight categories (Table 15.1). When attempting to diagnose a headache, there is no substitute for a thorough history and physical examination, but one should keep in mind certain “red flags” which may indicate a high suspicion for a secondary headache and the need for further workup and neuroimaging. The presences of these red flags has been shown in one study to correlate with abnormal neuroimaging.3 This study showed that papilledema, drowsiness, confusion, memory impairment or loss of consciousness, and paralysis were important clinical markers of central nervous system (CNS) pathology. A broad mnemonic to help remember these red flags is “SNOOP”: S—systemic signs or symptoms (e.g., fever); N—neurological signs or symptoms (e.g., partial paralysis); O—onset of a new or sudden headache; O—other associated conditions (e.g., headache is subsequent to head trauma, awakens patient from sleep, or is worsened by a Valsalva maneuver); and P—prior headache history (absence of prior headaches).4 Headache patients who have any of these red flags usually need further testing, starting with neuroimaging. In the following subsections we discuss some of the commonest and more dangerous secondary headaches.
Table 15.1 International Headache Society classification of secondary headaches
| Headaches attributable to |
| 1 Vascular disorder 2 Nonvascular intracranial disorder 3 Head and neck trauma 4 Infection 5 Disorders of the cranium, neck, eyes, ears, nose, sinuses, teeth, mouth, or other facial or cranial structures 6 Disorders of homeostasis 7 Substance or its withdrawal 8 Psychiatric conditions |
Subarachnoid Hemorrhage Headache
The typical patient with this problem is over 40 years of age. The subarachnoid hemorrhage headache (SAH) is often called “thunderclap headache” since it is a sudden, severe, generalized headache that reaches maximum intensity within 1 minute. The patient describes it as the “worst headache of my life.” This pain is secondary to leaking aneurysmal vessel bleeding beneath into the subarachnoid space. If the hemorrhage occurs within the cerebral or cerebellar tissues, it will be a rapid onset, severe, and deadly. Depending on the size of the ruptured vessel, the patient often presents with progressive loss of consciousness, vomiting, increasing pain symptoms, and clear neurologic deficits (i.e., hemiplegia or aphasia). Due to the pressure from the hemorrhage as well as pain-induced muscle spasm, the patient might experience nuchal rigidity and stiff neck. As the pressure increases, the patient my become semicomatose or fully comatose. Not all patients have full-blown aneurysmal rapidly progressive bleeds; some may have a sentinel bleed with less severe symptoms which can be followed with a more severe bleed in a few days.5 Vomiting can occur and increasing pain symptoms are often reported even though there are few, if any, neurologic signs of abnormality. The hemorrage can be seen on a computed tomographic (CT) scan, and further the leaking vessel can be identified with an arteriogram. Immediate medical assessment and treatment is essential to life.
Temporal Arteritis (Giant Cell Arteritis)
This headache usually involves one or both temporal regions, is moderate to severe, and is often associated with polymyalgia rheumatica.6 The headache may be generalized (polyarteritis nodosa) or localized (temporal arteritis). These headaches are localized in the area of the most severely affected arteries and are often described as a steady burning pain around the temples. Jaw claudication (fatigue) is pathognomic but uncommon. Age of onset is over 50 years in virtually all cases. Main criteria are (at least one of) the following: (1) swollen and tender scalp artery (usually superficial temporal artery); (2) elevated erythrocyte sedimentation rate (usually extremely high at around 100); (3) disappearance of headache within 48 hours of steroid therapy; (4) positive temporal biopsy showing giant cell arteritis.7 Temporal arteritis is a common form of this condition, and the patients are usually in their 60s or 70s. Typically, the arteries are elevated and tender to palpation and if there is severe artery occlusion and infarction (especially the ophthalmic artery) blindness will result.
Headache in Stroke Syndromes
Ischemic stroke patients present with less severe headache than patients with SAH. In fact headache is not commonly seen in lacunar (arterial) stroke patients and is a presenting symptom in 17–34% strokes, mostly posterior circulation ones.8 The typical stroke patient with headache is over 40 years old but some younger patients (especially female) have veno-occlusive disease.9 In addition to the headache, the patient will have clear neurologic deficits as a result of the infarction. The patient may present with vague head pains which are not severe. Headache is a much more common symptom of cerebral venous thrombosis and should be considered in every peripartum woman with or without other neurological signs or symptoms. Cerebral venous thrombosis may be accompanied by seizures and papilledema on neurological examination.
Brain Tumor Headache
Infratentorial brain tumors commonly present with headache in 80–85% cases.10 With the advent of advanced neruoimaging, headache is less often seen as an initial presenting symptom in brain tumors of other locations. Brain tumor headache is hard to distinguish from other musculoskeletal headaches because it may not have a specific characteristic and is typically described as a deep, aching, steady, dull pain.11 The headache may be severe but not usually as intense as migraines or cluster headaches. The “classic” brain tumor headache described as early morning headache is not common as an isolated symptom and is seen in less than 20% of cases. What distinguishes this headache from benign primary headaches is the associated neurological signs and symptoms: cognitive changes, focal neurological deficits, seizures, or signs of raised intracranial pressure that include worsening of headache with Valsalva.12
Meningitis Headache
The headache pains from meningitis are of rapid onset, severe, and associated with fever and signs of bacterial or viral infection.13 The patient often presents with an altered mental neurological deficit, vomiting, and increasing pain. Clinical findings of this disorder are a very stiff neck, limitation of straight leg rising, and a positive lumbar puncture with abnormal cerebral spinal fluid. The typical patient with meningitis is young, although anyone can get meningitis. Immediate medical management is essential to the patient’s recovery.
Secondary Brain Abscess
This condition occurs when bacteria enter the brain from an infection in an adjacent site such as the nasal and aural structures. Brain abscess causes fever, leukocytosis, and vomiting.14
Post-Traumatic Headache
This is a common secondary headache type that can be induced by mild-to-moderate closed head injury. Women have a 1.9-fold increased risk of post-traumatic headache (PTH) compared with men.15 Other risk factors include old age, position of head on impact (inclined or rotated), and previous history of headaches. Head trauma can trigger the onset of migraine headaches. In 85% of patient PTH resembles tension-type headache.16 This is a mild-to-moderate, deep aching headache which is often generalized, and worsened by even minimal physical or mental activity. Besides PTH, patients may have a variety of symptoms that constitute the spectrum of post-traumatic syndrome and include light-headedness, memory impairment, reduced attention span, inability to concentrate, anxiety, depression, and quick frustration. PTH onset occurs within 48 hours after the trauma, although delayed onset by several weeks is not uncommon. IHS criteria require that the headache onset should be within 2 weeks of head trauma to be classified as a post-traumatic headache. PTH is classified as major if loss of consciousness was significant, or if there is post-traumatic amnesia or at least two clinically abnormal neurologic signs. Complaints may persist for several months or even years.
15.1.B Episodic Headaches
There are many forms of episodic headaches, including episodic migraine (EM), probable migraine (PM) (a migraine subtype missing just one migraine feature), and episodic tension-type headache (ETTH).
Episodic Tension-Type Headaches
This is the most frequent type of primary headache and lifetime prevalence in the general population ranges from 30% to 78%. Schwartz reported 1-year prevalence of 38% of ETTH in the United States population with preponderance in women.17 The 1-year prevalence of TTH is much higher in Denmark at 84.7%.18 In this study, tension-type headaches were divided into infrequent episodic, frequent episodic, and chronic tension-type headache, and the prevalence for each was also reported (48.2%, 33.8% and 2.3%, respectively). There was female preponderance and self-reported migraine was a risk factor for frequent episodic and chronic tension-type headache. These figures were confirmed by a study of tension-type headaches in twin pairs that was examining to see if genetic factors were important.19 This study recruited twin pairs from the population-based Danish Twin Registry. A total of 3523 monozygotic 4150 dizygotic same-gender and 3526 dizygotic opposite-gender twin pairs were included. The authors reported that the prevalence of infrequent episodic headache was 68% in men and 66% in women. More important for this discussion, the prevalence of frequent episodic headache was 9% in men and 24% in women. ETTHs are usually described as “tight hat band headache” and maybe associated with pericranial tenderness involving the head and neck muscles.20 Even though this is the most frequent type of headache, the symptoms may be nonspecific. The International Headache Society has specific criteria for making this diagnosis (Table 15.2). Headache duration may vary from short, to that lasting hours, and may increase slowly during the day to reach peak intensity near late afternoon.21
Table 15.2 Episodic tension-type headache (ETTH)
|
Infrequent ETTH A At least 10 episodes occurring on less than 1 day per month (<12 days per year) and fulfilling criteria B–D Frequent ETTH A At least 10 episodes occurring on ≥1 but <15 days per month for at least 3 months (≥12 and <180 days per year) and fulfilling criteria B–D |
*See ref. 1 (Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders, 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160).
Episodic Migraine with or Without Aura
Migraine headaches affect 2 million Americans annually and account for over $30 billion in lost productivity.22 The prevalence of migraine in Western countries is 12–16% and is highest in persons aged 25–55 years. Migraines occur slightly in more women than men. The peak of onset of migraine without aura in men is 10–11 years and for women 14–17 years. Migraine with aura peaks at an earlier age. Approximately 66% of headache pain in the elderly is caused by either migraines or tension-type headaches and this figure is well over 90% in a younger cohort.23 The good news is that the overall prevalence of headaches declines with age and in fact it has been reported that the number of headaches declines from 83% of individuals between 21 and 34 years to 59% between ages 55 and 74.24 One exception to this is that migraines sometimes occur for the first time after age 50; about 2% of all migraines start at this late age.25 A large epidemiologic study was reported in 2000 which described headache prevalence in Norway.26 These authors reported the 1-year prevalence for migraine was 12% (16% in women and 8% in men). They also reported the prevalence for chronic daily headache (>14 days per month) as 2%, and while this figure may include other forms of chronic headache, many were due to converted episodic migraine. Migraine is not simply a headache; it is a syndrome that comprises emotional, psychological, and neurological symptoms.27 Migraines are typically episodic headaches with much greater severity than ETTH. The main difference is that patients with ETTH pain often continue to work while migraine pain will be aggravated by or cause avoidance of normal activities. The International Classification of Headache Disorders (2nd revision) divides migraine into six categories (Table 15.3).
Table 15.3 International Headache Society–based migraine categories
| 1 Migraine 1.1 Migraine without aura 1.2 Migraine with aura 1.2.1 Typical aura with migraine headache 1.2.2 Typical aura with nonmigraine headache 1.2.3 Typical aura without headache 1.2.4 Familial hemiplegic migraine (FHM) 1.2.5 Sporadic hemiplegic migraine 1.2.6 Basilar-type migraine 1.3 Childhood periodic syndromes that are commonly precursors of migraine 1.4 Retinal migraine 1.5 Complications of migraine 1.5.1 Chronic migraine 1.5.2 Status migrainosus 1.5.3 Persistent aura without infarction 1.5.4 Migrainous infarction 1.5.5 Migraine-triggered seizure 1.6 Probable migraine |
Migraine headaches have a set of defining criteria set forth by the International Headache Society (Table 15.4). Migraine aura is present in only 20% of patients, is usually a visual phenomenon, and is typically described as a “flashing light or dizziness.” A migraine headache typically occurs within 60 minutes of the onset of the aura. There are several migraine variants, such as hemiplegic migraine (head pain, transient motor–sensory changes), ophthalmoplegic migraine (eye pain, transient optic nerve palsy with diplopia–ptosis), migrainous infarction (cerebral vascular ischemia with infarction and cerebral tissue damage), and midface migraine (orodental pain, 4–72 hours, nausea, vomiting, phonophobia, and photophobia).
Table 15.4 International Headache Society criteria for migraine without aura
| A At least five episodes fulfilling criteria B–D B Headache lasting from 4 to 72 hours (untreated or unsuccessfully treated) C Headache has more than two of the following characteristics: 1 Unilateral location 2 Pulsating quality 3 Moderate or severe intensity 4 Aggravated by or causing avoidance of routine physical activity D During headache one or more of the following: 1 Nausea and /or vomiting 2 Phonophobia and photophobia E Not attributed to another disorder |
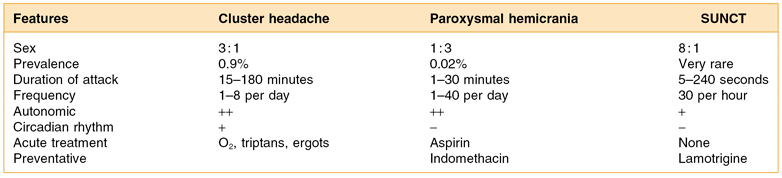
Cluster Headaches and Other Trigeminal Autonomic Cephalalgias
While far less common than migraine or tension headache, the trigeminal autonomic cephalalgias (TACs; cluster headaches, paroxysmal hemicranias, and SUNCT) must be described also.28 Cluster headaches (CHs) are a rapid-onset, intense paroxysmal one-sided orbital, supraorbital, and temporal pain lasting 15–180 minutes when untreated (Table 15.5). The incidence is 1 in 1000. In CH, the afflicted are mostly men (5–6 times greater than women), heavy smokers and drinkers, with age of onset, 20–40 years.29 A typical leonine face with deep nasal furrows, scant eyebrows, and skin changes that include peau d’orange and telangectasias has also been described with these patients. With a cluster headache, the patients are very agitated during the attack (pacing and head pounding) and have no preheadache aura and usually no associated nausea or vomiting. Attacks can be precipitated by alcohol, histamine, or vasodilators. The CHs will often repeat several times in a 24-hour period (1 attack every other day to as many as 8 per day). The headaches often occur at night, and attacks wake patients usually within 60–90 minutes after falling asleep. Recent studies report that up to 80% of CH patients have obstructive sleep apnea. The cluster period frequently lasts for weeks to months and is usually present in specific seasons of the year (more in winter and spring) with months of remission. CH is classified as episodic but there is a chronic subform based on the length of remitting period (episodic, remission period of 1 month or longer between cluster periods; chronic, attacks last over 1 year or remitting period shorter than 1 month in length). Women have shorter duration clusters, fewer autonomic symptoms, less miosis. Migrainous symptoms are commoner. There is a strong genetic predisposition to this disease; there is a 14-fold increased risk of CH in first-degree relatives. Family history is positive in 11% cases of CH. Recently polymorphism of the hypocretin receptor 2 gene (responsible for narcolepsy) was found to be associated with CH, and there is a fivefold increased risk of CH in homozygotes.30 Interestingly hypocretin-secreting cells are highly concentrated in the hypothalamus, and new data suggests dysfunction of hypothalamus in cluster headaches.
Table 15.5 Cluster headache criteria
| A Severe, unilateral, supraorbital, and/or temporal pain lasting 15–180 minutes B Headache accompanied by at least one of the following ipsilaterally: 1 Conjunctival injection and/or lacrimation 2 Nasal congestion and/or rhinorrhea 3 Miosis and/or ptosis 4 Eyelid edema 5 Forehead and facial sweating 6 Sense of restlessness or agitation C Frequency of attacks: 1–8 per day |
Paroxysmal Hemicrania
This even rarer TAC-type headache disorder affects mostly women (the female-to-male ratio is around 2:1).31 Prevalence is unknown and is estimated to be around 1 in 50,000. The pain occurs as a sharp, intense pain and is often described as a breath-taking, “stop-what-you’re-doing” immediate pain. The symptoms of paroxysmal hemicrania (PH) involve shorter and more frequent pain events each day than in cluster pain. There must be 20 or more headaches per day (Table 15.6). Unlike CH, PH patients do not have seasonal occurrence and each pain event is about 5–20 minutes. In a 24-hour period there will be 10–30 pain events. Nausea and vomiting are occasionally seen; the patient can be awakened from sleep, but this is not the typical presentation. In PH, the pain symptoms are usually localized to temple, forehead, ear, eye, or occipital regions and autonomic symptoms (flushing, rhinorrhea) are similar to cluster headache. A unique feature of PHs is that they are almost always responsive to indomethacin (150 mg/day). PH is also classified as episodic and chronic subform based on the length of remitting period (same as with cluster headache).
Table 15.6 A comparison of trigeminal autonomic cephalalgias
SUNCT, short-lasting unilateral neuralgiform headache with conjunctival injection and tearing.
Short-Lasting Neuralgiform Headache with Conjunctival Injection and Tearing (SUNCT)
This syndrome was described by Sjaastad and is the rarest of all TACs. There are only 30 documented cases worldwide.32 It is characterized by short-lasting attacks of unilateral orbital, supraorbital, or temporal pain that are much briefer than those seen in CH or PH. Pain must be accompanied by ipsilateral conjunctival injection and lacrimation. Attacks of stabbing or pulsating pain last 5–240 seconds and occur with a frequency from 3 to 200 per day. Treatments that have been used successfully in patients include intravenous lidocaine 4 mg/min, carbamazepine 1200 mg, lamotrigine 200 mg, topiramate 200 mg, and gabapentin 2400 mg.
15.1.C Other Primary Headaches
Some of the headaches in this category are induced by a specific activity (e.g., cough or exertion) and therefore these headaches have been named after their inducing activity. There is usually no specific treatment for this category and etiopathogenesis remains puzzling. A list of the conditions in this group are seen in Table 15.7.
Table 15.7 Other primary headaches
| 1 Primary cough headache 2 Primary exertional headaches 3 Primary headache associated with sexual activity 4 Hypnic headaches 5 Primary thunderclap headache 6 Primary stabbing headache (“jolts” and “jabs”): This disorder presents as sudden and sharp and lasts only for seconds to a few minutes; it affects more females than males. It is common in a migraine population, but it may occur as a primary manifestation in some, especially those above the age of 60. This condition is frequently responsive to indomethacin (150 mg or less daily). |
15.2 Suggested Etiologies and Mechanisms for Episodic Headaches
15.2.A Etiology of Tension-Type Headaches
Of course there are many theories that are put forth to explain the causation and pathogenesis of ETTH. An important issue we must discuss, and one that is moderately controversial, is the role that pericranial muscle and fascial tenderness play in the causation or triggering of ETTH. The questions that need addressing are twofold:
1 “Does jaw or facial muscle tension cause an ETTH?”
2 “If muscle tension is not causative, does muscle nociception from the jaw, face, and neck potentially assist in the triggering process for ETTHs and migraines?”
These issues are important because later, in the section on treatment, the role that myofascial pain and local myalgia play is critical to the overall headache management program.
Jaw Muscle Activity As an Etiologic Factor for Episodic Headaches
Regarding the first question in Section 15.2.A, some argue that pericranial tenderness is evidence that elevated substantial muscle tension levels are causative of ETTH. On this point, the data are actually quite clear that the presence of strong habitual or involuntary motor contraction as a precursor to headache is not correct. In 1995 a study documented the relationship between stress, pain, physical activity, and temporalis muscle electromyography (EMG) in 36 tension-type headache patients and 36 age- and sex-matched controls.33 Every 30 minutes EMG level, pain intensity, stress, and physical activity levels were recorded in a daily diary for a 3-day period. A time-lagged cross-correlational analysis between pain, stress, physical activity, and EMG showed that the highest correlation coefficient values occurred between pain and stress at the same (r = 0.33) and at the two preceding 0.5-hour time points (r = 0.21 and r = 0.26) in the headache group, suggesting that stress precedes the headache in many cases. The study found virtually no correlation between pain, stress, or physical activity with temporalis muscle EMG for either group. Unfortunately, the subjects in this study were not self-acknowledged “tooth clenchers” and, moreover, wearing an EMG recording unit and stopping to record their levels every 30 minutes in a diary may have interfered with any oral habit, even if present. Some of the recent data on tooth clenching suggests that a stronger correlation does exists between tooth clenching and myofascial pain than ETTH, but this is an still unproven causal relationship (see Chapter 19 for a detailed discussion of oral motor disorders). Nevertheless, the data reported by Clark et al.33 suggested that temporalis muscle activity levels were not related to the rise and fall of the subjects’ headache pain or stress levels. Conversely, elevated stress did appear to be related to headache pain. A further analysis of these data in 1997 looked at the subjects’ collected cumulative temporalis muscle activity.34 The authors reported that neither the waking nor the sleeping overall muscle activity levels for these two groups were statistically different. However, when the waking EMG data were dichotomized into functional and nonfunctional activities, a significant difference was found between groups during jaw function (i.e., chewing and talking). These data suggest that headache subjects are using their temporalis muscles with less efficiency than nonheadache subjects during function and the authors concluded that this elevated EMG is more likely a consequence of pain (via protective splinting or guarding) rather than a cause in tension-type headache sufferers.
Myofascial Pain As a Trigger for Episodic Headache
Regarding the second question posed at the beginning of Sec 15.2.A, few suggest that clenching plays a prominent role in the genesis of migraine, but several studies have suggested clenching may increase the likelihood that patients will have more myofascial pain and this nociceptive process may then trigger both migraine and tension-type headache events.35 For example, in 2006, the findings above were confirmed by a study that examined stress-induced pain and muscle activity in patients with migraine and tension-type headache.36 This study recorded pain and surface electromyography (EMG) from the neck and jaw muscles in 22 migraineurs during headache-free periods, 18 patients with tension-type headache (TTH), and 44 healthy controls. Recordings were made during both a 60-minute experimental cognitive stress task and a 30-minute relaxation period in the laboratory. The authors reported that TTH patients had higher pain reports in the temporalis and frontalis regions than neck region (trapezius and splenius) but EMG responses were not different from controls in headache patients, and EMG responses did not correlate with pain responses.
In 1999 a pathophysiological mechanism for tension-type headache was offered37,38: (1) tension-type headache was the most prevalent form of headache, with a lifetime prevalence of 78% in a general adult population; (2) 30% were affected more than 14 days per year and 3% were chronically affected (i.e., had headache at least every other day); (3) females were more frequently affected and were more tender on palpation than males, and young subjects more frequently affected and more tender on palpation than older subjects. Substantially more pericranial muscle tenderness was found in subjects with tension-type headache compared with migraineurs or control nonheadache patients. Tenderness increased significantly with increasing frequency of tension-type headache in both males and females. Subjects with chronic tension-type headache had slightly increased EMG levels during resting conditions. In a subsequent clinical, controlled study, the effect of 30 minutes of sustained tooth clenching was studied; within 24 hours, 69% of patients and 17% of controls developed a tension-type headache. Likewise, psychophysical and EMG parameters were studied in 28 patients with tension-type headache, both during and outside of a spontaneous episode of tension-type headache. It was concluded that a peripheral trigger for tension-type headache is possible but it is most likely in the episodic subform, whereas a secondary, segmental central sensitization and/or an impaired supraspinal modulation of incoming stimuli seems to be involved in subjects with chronic tension-type headache. Prolonged nociceptive stimuli from myofascial tissue may be of importance for the conversion of episodic into chronic tension-type headache. In summary, the authors emphasize that tension-type headache is a multifactorial disorder with several concurrent pathophysiological mechanisms, and that extracranial myofascial nociception may constitute only one of them. The pericranial muscle tenderness and abnormal EMG activity that are observed in these patients are independent of the headache; on the one hand, there is abnormal pain sensitivity due to supraspinal facilitation and, on the other, there is ineffective antinociception.
15.2.B Pathogenesis of Migraine
The etiology of migraine is thought be related to central neurologic excitability and there clearly is a genetic basis to this disease.39,40 Current research supports the trigeminovascular theory to explain the pathogenesis of migraine. According to this theory, there is baseline neuronal hyperexcitability, which in aura patients leads to cortical spreading depression that ultimately triggers a wave of sterile inflammatory neurochemical due to which there is the final pathway of vasoconstriction and vasodilatation and head pain.41 Positron emission tomographic (PET) studies have clearly shown the brain stem as the generator for migraine, with activation of the contralateral pons during acute migraine.42
15.2.C Pathogenesis of Trigeminal Autonomic Cephalalgias
Recent studies have shown that the hypothalamus is the generator of cluster headaches and is responsible for stimulation of the parasympathetic and sympathetic pathways and the trigeminal vascular system.43 Matharu et al. have also shown that the hypothalamus may play an equally important role in HC and CPH and therefore the TACs as a group may end up having a common neuroanatomic basis.44,45
15.3 Episodic Headache Treatment
There are multiple modalities of treatments available for episodic headache, including over-the-counter medications, stress reduction, myofascial-based therapy, triggering factor avoidance, behavioral therapies, physical medicine modalities, trigger point and other injections, and of course stronger abortive medications such as the class of serotonin modulators called triptans and ergots. The efficacy evidence for these medications and procedures is discussed below.
15.3.A Over-The-Counter Medications
Normally a brief, nondisabling, ETTH is not enough of a problem for a patient to seek a medical or dental consultation, that is, unless it becomes quite frequent or is severe in intensity. In most cases patients with mild to moderate and infrequent episodic headaches, occurring only a few times a month, are managed with over-the-counter analgesics, or a bite guard appliance for those who suspect habitual clenching of the teeth as a cause. When the headache is not “frequent,” over-the-counter (OTC) medications available include acetaminophen, aspirin, ibuprofen, and naproxen, and all can be an effective method of relieving the headache. While some data shows that nonsteroidal anti-inflammatory drugs (NSAIDs) may be more effective than acetaminophen and aspirin for headache management, there is no strong evidence to suggest that one is consistently better than the others.46 Costwise, this is a very inexpensive therapy compared with many prescription medications.47
The problem arises with more severe or more frequent headaches. One such problem resulting from frequent use of analgesic medications is a medication overuse headache (MOH), which will become much more difficult to treat.48 Alternatively, patients with frequent or severe headaches do start consulting their physicians about the problem and then they are given a stronger medication such as opioid-class drugs (hydrocodone or codeine) or drugs with barbiturate properties (e.g., Fiorinol or Fioricet). In general, these latter drugs are not recommended as appropriate for frequent headaches since they have dependence, tolerance, and paradoxically sometimes even antianalgesic properties with ongoing use.
15.3.B Headache Avoidance Checklist
Episodic tension-type headaches (ETTH) are usually triggered by stress, but like migraines, they may have other triggers including behavioral and psychiatric.49 For this reason, it is always prudent to give the patient a headache avoidance checklist to follow. The patient should also be given a headache calendar to record each episode of headache and the medications taken, along with any possible triggers such as food or activity prior to headache. Some well-known food triggers include old cheese, red wine, certain food additives (e.g., aspartame and MSG). This checklist has several elements (Table 15.8).
Table 15.8 Episodic headache avoidance checklist
| 1 Diet instructions (e.g., do not miss meals)—hypoglycemia triggers migraine and ETTH. 2 Wear sunglasses outside—bright light simulation triggers migraine. 3 Ice packs applied where the headache hurts—cooling decreases pain nerve activity. 4 Posture awareness—instruct the patient to watch neck posture so that the cervical spine is in a neutral position. 5 Ergonomic changes to workspace—it is important to have students and office workers raise the level of their desk so the neck is not as flexed; use a speakerphone, and avoid bending the neck when using the telephone. 6 Daily stretching of neck muscles—every 2 hours perform neck stretching exercises to reduce nociceptive activity and tension in neck. 7 Alcohol, tobacco, and caffeine avoidance or moderation—these drugs can trigger headache; however, if the patient is a daily user of these agents, abrupt cessation of these drugs can trigger a withdrawal headache, so in this case moderation of drug use is appropriate. Note: Cluster headaches can be triggered by smoking, alcohol, and vasodilators such as nitroglycerine. Unfortunately, the other TACs are not usually triggered by easily avoidable environmental factors. |
ETTH, episodic tension-type headache; TACs, trigeminal autonomic cephalalgias.
15.3.C Myofascial-Based Treatment for Headache
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


