Chapter 14
Dental materials
Contents
Principal sources J. F. McCabe 2008 Applied Dental Materials, Blackwell Publishing.
Properties of dental materials
Definitions
Coefficient of thermal expansion
The fractional ↑ in length for each degree of temperature ↑.
Creep
The slow plastic deformation that occurs with the application of a static or dynamic force over time.
Elastic modulus
A measure of the rigidity of a material, defined by the ratio of stress to strain (below elastic limit).
Fatigue
When cyclic forces are applied, a crack may nucleate and ↑ by small increments each time the force is applied. In time the crack will ↑ to a length at which the force results in # through the remaining material.
Hardness
Resistance to penetration. A number of hardness scales are in use (e.g. Vickers, Rockwell). Between these scales hardness values are not interchangeable.
Resilience
The energy absorbed by a material undergoing elastic deformation up to its elastic limit.
Stiffness
An indication of how easy it is to bend a piece of material without causing permanent deformation or #. It is dependent upon the elastic modulus, size, and shape of the specimen.
Strain
Change in size of a material that occurs in response to a force. It is the change in length divided by the original length.
Stress
Internal force per unit cross-sectional area acting on the material. Can be classified according to the direction of the force: tensile (stretching), compressive, or shear.
Wear
The abrasion (mechanical or chemical) resistance of a substance.
Thermal conductivity
Ability of a material to transmit heat.
Thermal diffusivity
Rate at which temperature changes spread through a material.
Toughness
The amount of energy absorbed up to the point of #. A function of the resilience of the material and its ability to undergo plastic deformation rather than #.
Wettability
Ability of one material to flow across the surface of another, determined by the contact angle between the two materials and influenced by surface roughness and contamination. The contact angle is the angle between solid/liquid and liquid/air interfaces measured through the liquid.
Yield strength
(or elastic limit) The stress beyond which a material is permanently deformed when a force is applied.
Evaluation of a new material
Before it reaches the dental supply companies a new material should have undergone the following tests:
Clinically, the important questions to ask the rep include:
Then decide whether this new material has any significant advantages over the material you are familiar with. Help can be sought from user ratings bodies such as Reality ( www.realityesthetics.com).
www.realityesthetics.com).
‘Be not the first by whom the new are tried, nor yet the last to lay the old aside.’ Alexander Pope.
Restoration of teeth
When restoring or replacing teeth it should be remembered that the teeth themselves are composed of enamel, dentine and pulpal tissues each with quite different properties that must be taken into account when selecting a material with which to restore them. In addition the teeth emerge from a complex dentogingival junction and are supported in alveolar bone by the viscoelastic periodontal ligament. All of these features are difficult to reproduce with current dental materials and most likely will not be until teeth themselves are able to be bioengineered.
Amalgam
An amalgam is a mixture of Hg and another metal. Dental amalgam is made by mixing together Hg with a powdered silver–tin alloy (mercury around 50% by weight) to produce a plastic mass that can be packed into a preparation before setting. Despite toxicity scares and the introduction of posterior composites, amalgam is still widely used, mainly because of its ease of handling. In Norway, Denmark and Sweden dental amalgam is banned for environmental reasons.
Types of amalgam
There are two ways of classifying amalgam:
Particle shape
Can be lathe-cut (irregular), spheroidal, or a mixture of the two. Spheroidal particles give a more fluid mix which is easy to condense, can be carved immediately, and take 3h to reach occlusal strength (compared to >6h for lathe-cut amalgams). Spheroidal amalgams are preferable for pinned restorations.
Particle composition
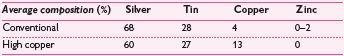
The first (conventional) alloys introduced had a low copper content (5%). The weakest (Sn–Hg or γ-2) phase of the set amalgam could be eliminated by ↑ the proportion of copper, so a variety of high copper (10–30%) amalgams have been introduced that react to eliminate it. These are more expensive, but superior in terms of corrosion resistance, creep, strength, and durability of marginal integrity. There are two types of high-copper alloy: (1) a single composition alloy of silver–tin–copper; (2) a blended (dispersion) mix of silver–tin and copper–silver alloys. Of these, (1) is the most resistant to tarnishing.
Handling characteristics
Mixing or trituration
This is carried out mechanically, in one of two ways:
Condensation
Carry out incrementally by hand instruments (lathe-cut or spheroidal). Preparations should be overfilled so that the Hg-rich surface layer is removed by carving.
Carving
With spherical alloys this can be commenced immediately, but with lathe-cut a delay of a few minutes is advisable. Burnishing is now back in vogue.
Polishing
Polished amalgams look good, but whether polishing is necessary is still the subject of debate. NB Maximum strength takes 24h to develop.
Marginal leakage
Whilst amalgam corrosion products will form a marginal seal in time, microleakage can be ↓ by the use of either a conventional cavity varnish (e.g. Copalite®) or a bonding agent (e.g. Amalgambond® or Panavia-21™). The latter (an anaerobic resin adhesive) also bonds to set amalgam.1 Alternatively, sealing over the completed restoration with a fissure sealant has been suggested, and this is a possible solution to the ‘ditched’, but caries-free amalgam.
Toxicity
‘There is insufficient evidence to justify the claim that Hg from dental amalgam has an adverse effect on the vast majority of patients’.2 However, current advice includes avoiding the placement of amalgam fillings during pregnancy.3 In addition there does seem to be a link between allergy to constituents of dental amalgam and lichenoid eruptions in the oral cavity.
The greatest risk appears to be related to the inhalation of Hg vapour and as such the dental team as well as patients is theoretically at risk;
Attention should be paid to the following:
Types of amalgam currently available
Conventional lathe-cut, conventional spherical, high-copper dispersion lathe + spherical, high-copper single spheroidal, high-copper single lathe-cut.
Composite resins—constituents and properties
The modern composite resin is a mixture of resin and particulate filler, the handling characteristics of which are determined largely by the size of the filler particles and method of cure.
Constituents
Resin
Most composite resins are based on either Bis-GMA (addition product of bisphenol A and glycidylmethacrylate) or urethane dimethacrylate plus a diluent monomer, triethylene glycol dimethacrylate (TEGMA).
Filler
(e.g. quartz, fused silica, glasses such as aluminosilicate and borosilicate). Confers the following benefits on the composite resin:
Composite resins can be subdivided according to particle size:
Macrofilled
(or conventional) Contains particles of radio-opaque barium or strontium glass 2.5–5μm in size, to give 75–80% by weight of filler. Good mechanical properties, but hard to polish and soon roughens.
Microfilled
Contains colloidal silica particles 0.04μm in size and 30–60% by weight. Retains a good surface polish, but is unsuitable for load-bearing situations, has poor wear resistance, and ↑ contraction shrinkage.
Nanofilled
By combining nanometric particles and nanoclusters in a conventional resin matrix manufacturers claim to offer ↑ wear resistance as well as polishability and lustre.
Hybrid
Contains a mixture of conventional and microfine particles designed to optimize both mechanical and surface properties. Contains 75–85% by weight of filler, of which the bulk is conventional (1–50μm). Some manufacturers achieve up to 90% filler loadings by using blended sizes of filler particles.
Initiator/activator
Other constituents
include pigments, stabilizers, and silane coupler to produce bond between particles and filler.
Composites may also be classified as:
Important properties of composites
Newer developments include hydroxyl ion releasing composite resin to counteract demineralization during periods of low pH in the restoration. Also use of fibre reinforcement to fabricate posts and cores as well as bridges/splints.
Composite resins—practical points
Method of polymerization
Chemical (self-cure)
No additional equipment required, but mixing of two components introduces porosity and the working time is limited.
Light activation
Provides long working time, command set, and better colour stability, but requires a light source, has a limited depth of cure, and the temperature ↑ during setting can be as high as 40°C. Three types of light source are currently available: quartz tungsten halogen (QTH), plasma arc light (PAC), and light-emitting diode (LED). QTH is the most widely used although the newer PAC and LED lights confer theoretical advantages in e.g. speed and depth of cure, portability, lifetime and reliability of light source. Ramping of intensity of light emission confers the theoretical benefit of less polymerization shrinkage.
Dual-cure
Curing is initiated by a conventional light source, but continues chemically to help ensure polymerization throughout the restoration.
Practical tips for light activation
Finishing
Ideally, a mylar strip to produce the contour of the restoration. Then refine with microfine diamond or multi-bladed tungsten carbide finishing burs (under water spray), finishing strips, and then polish with aluminium oxide-coated discs (Shofu, Soflex). Shofu points or finishing pastes are useful for inaccessible concave surfaces.
Problems with composite resins
Indirect composite inlays may circumvent the last two problems (p. 232).
Fissure sealants
Composite resins containing little or no filler, which are either self- or light-cured. Clear or opaque types are available, the former having better flow characteristics (whether this is an advantage depends upon the position of the tooth). Success depends upon being able to achieve good moisture control for the acid-etch bond.
Flowable composite resins
are now available. They are predominately resin with a reduced percentage of filler particles, and consequently shrink considerably on curing. Some advocate using them as liners or in the bottom of proximal preparations, but the high shrinkage precludes this. RMGIC is preferable in proximal preparations, which are below the cemento-enamel junction (bonded-base approach); however, they have a place in the marginal repair of restorations and some advocate their use for facial cervical margin restorations.
Acid-etch technique
Recent research would suggest that:
| Etched zone (enamel removed) | 10μm |
| Qualitative porous zone | 20μm |
| Quantitative porous zone | 20μm |
Composite resin tags may penetrate up to 50μm into enamel to give micromechanical retention.
NB Many of the newer dentine adhesive systems do not have a separate acid-etch stage. These systems use a combination of acidic primers and bonding resins, either as a single stage or applied separately (Adper™ Prompt™ L-Pop™, iBond®, Xeno IV®). (See dentine bonding section, p. 616)
Dentine-adhesive systems (dentine bonding agents)
The advantages of bonding to dentine (e.g. preservation of tooth tissue) have fuelled considerable research effort. The problems that have had to be overcome include the high water and organic content of dentine; the presence of a ‘smear layer’ after dentine is cut; and the need for adequate strength immediately following placement, to withstand the polymerization contraction of composite resin. These difficulties have been approached in a number of ways, making the topic of dentine bonding confusing, a situation which has been exacerbated by the pace of new developments and by the claims of the manufacturers.
Indications
The smear layer
consists of an amorphous layer of organic and inorganic debris, produced by cutting dentine. It ↓ sensitivity by occluding the dentine tubules and prevents loss of dentinal fluid. The smear layer is partially or completely removed &/or modified during dentine bonding.
Mechanism of dentine bonding
Most dentine adhesive systems aim to modify and partially remove the smear layer, by the application of an acidic primer. This demineralizes the underlying surface, exposing the collagen and opening up the dentinal tubules. It is important to keep this surface moist to prevent the collagen becoming flattened (not with saliva, though!). This layer is then infiltrated using a resin with bi-functional ends: one hydrophilic end, which is able to bond to wet dentine and one hydrophobic end capable of bonding to the composite resin. In this infiltrated hybrid layer molecular entanglement of the collagen and resin occurs, providing the basis for the bonding system.
Practical points
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


