N
Drug Class:
Mechanism of Action
An NSAID that produces analgesic and antiinflammatory effects by inhibiting prostaglandin synthesis.
Therapeutic Effect: Reduces the inflammatory response and intensity of pain.
Serious Reactions
! Overdose may result in acute hypotension and tachycardia.
! Rare reactions with long-term use include peptic ulcer disease.
! GI bleeding, gastritis, nephrotoxicity (dysuria, cystitis, hematuria, proteinuria, nephrotic syndrome), severe hepatic reactions (cholestasis, jaundice), and severe hypersensitivity reactions (bronchospasm, angioedema).
Dental Considerations
General:
• Potential increase of adverse cardiovascular events in patients at risk for thromboembolism.
• Patients on chronic drug therapy may rarely have symptoms of blood dyscrasias, which can include infection, bleeding, and poor healing.
• Assess salivary flow as a factor in caries, periodontal disease, and candidiasis.
• Avoid prescribing in pregnancy.
• Avoid prescribing aspirin-containing products.
• Consider semisupine chair position for patients with arthritic disease.
• Severe stomach bleeding may occur in patients who regularly use NSAIDs in recommended doses, when the patient is also taking another NSAID, a blood thinning, or steroid drug, if the patient has GI or peptic ulcer disease, if they are 60 yr or older, or when NSAIDs are taken longer than directed. Warn patients of the potential for severe stomach bleeding.
Drug Class:
Nonselective β-adrenergic blocker
Indications and Dosages
Side Effects/Adverse Reactions
Nadolol is generally well tolerated, with transient and mild side effects
Drug Interactions of Concern to Dentistry
• Sympathomimetics (epinephrine, norepinephrine, isoproterenol): elevated systolic blood pressure, bradycardia or cardiac arrest (limit or avoid vasoconstrictors)
• Slows metabolism of nadolol: lidocaine
• Increased hypotension, myocardial depression: fentanyl derivatives, hydrocarbon inhalation anesthetics
• Decreased hypotensive effect: indomethacin and other NSAIDs
Serious Reactions
! Overdose may produce profound bradycardia and hypotension.
! Abrupt withdrawal of nadolol may result in diaphoresis, palpitations, headache, tremors, exacerbation of angina, MI, and ventricular arrhythmias.
! Nadolol administration may precipitate CHF and MI in patients with cardiac disease; thyroid storm in those with thyrotoxicosis; and peripheral ischemia in those with existing peripheral vascular disease.
! Hypoglycemia may occur in patients with previously controlled diabetes.
Dental Considerations
General:
• Monitor vital signs at every appointment because of cardiovascular side effects.
• Patients on chronic drug therapy may rarely have symptoms of blood dyscrasias, which can include infection, bleeding, and poor healing.
• After supine positioning, have patient sit upright for at least 2 min before standing to avoid orthostatic hypotension.
• Limit use of sodium-containing products, such as saline IV fluids, for patients with a dietary salt restriction.
• Assess salivary flow as a factor in caries, periodontal disease, and candidiasis.
• Stress from dental procedures may compromise cardiovascular function; determine patient risk. Short appointments and a stress-reduction protocol may be required for anxious patients.
• Consider semisupine chair position for patients with respiratory distress.
Consultations:
• In patients with symptoms of blood dyscrasias, request a medical consultation for blood studies and postpone dental treatment until normal values are reestablished.
• Take precautions if dental surgery is anticipated and anesthesia is required.
• Medical consultation may be required to assess disease control and patient’s ability to tolerate stress.
Drug Class:
Drug Class:
Category and Schedule
Pregnancy Risk Category: B (D if used for prolonged periods or at high dosages at term)
Drug Class:
Opioid agonist, antagonist; opioid analgesic
Uses
Relief of moderate-to-severe pain, preoperative sedation, obstetric analgesia, adjunct to anesthesia
Serious Reactions
! Abrupt withdrawal after prolonged use may produce symptoms of narcotic withdrawal, such as abdominal cramping, rhinorrhea, lacrimation, anxiety, fever, and piloerection (goose bumps).
! Overdose results in severe respiratory depression, skeletal muscle flaccidity, cyanosis, and extreme somnolence progressing to seizures, stupor, and coma.
! Repeated use may result in drug tolerance and physical dependence.
Dental Considerations
General:
• Avoid use in an opioid-dependent patient.
• Acute-use drug; question patient about use for pain.
• If additional analgesia is required for dental pain, consider alternative analgesics (NSAIDs) in patients taking opioids for acute or chronic pain.
• Monitor and record vital signs.
• Assess salivary flow as a factor in caries, periodontal disease, and candidiasis.
Teach Patient/Family to:
• Encourage effective oral hygiene to prevent soft tissue inflammation.
• Prevent trauma when using oral hygiene aids.
• Avoid driving or other activities requiring mental alertness.
• Avoid alcohol ingestion or CNS depressants; serious CNS depression may result.
• Avoid OTC preparations that contain CNS depressants (antihistamines, cold remedies).
Drug Class:
Indications and Dosages
Side Effects/Adverse Reactions
CNS: Dizziness, headache, dysphoria, perception of pain, nervousness
CV: Tachycardia, hypertension, dysrhythmia, hypotension
GI: Nausea, abdominal cramps, vomiting, diarrhea
Serious Reactions
Dental Considerations
General:
• This drug is intended for acute use only.
• Risk of seizures reported in animal studies; be aware of this potential.
• Serious cardiovascular events have been associated with opioid reversal in postoperative patients; doses should be carefully titrated to reduce these events.
• Buprenorphine depression may not be completely reversed.
• In all cases, the establishment of a patent airway, ventilatory assistance, oxygen administration, and circulatory access should complement or precede opioid antagonist use.
• Significant opioid depression occurring in the dental office may require relocation of the patient to a medical facility for comprehensive management.
• Patients discharged from the office or emergency facility should be carefully observed for the return of opioid-induced depression.
Drug Class:
Mechanism of Action
An opioid antagonist that displaces opioids at opioid-occupied receptor sites in the CNS.
Uses
Treatment of respiratory depression induced by opioids, to reverse postoperative opioid depression
Indications and Dosages
Side Effects/Adverse Reactions
None known; little or no pharmacologic effect in absence of narcotics
Serious Reactions
! Too-rapid reversal of opioid-induced respiratory depression may result in nausea, vomiting, tremors, increased B/P, and tachycardia.
! Excessive dosage in postoperative patients may produce significant excitement, tremors, and reversal of analgesia.
! Patients with cardiovascular disease may experience hypotension or hypertension, ventricular tachycardia and fibrillation, and pulmonary edema.
Dental Considerations
General:
• This drug is indicated for acute use only.
• Risk of seizures reported in animal studies; be aware of this potential.
• Serious cardiovascular events have been associated with opioid reversal in postoperative patients; doses should be carefully titrated to reduce these events.
• Buprenorphine depression may not be completely reversed.
• In all cases, the establishment of a patent airway, ventilatory assistance, oxygen administration, and circulatory access should complement or precede opioid antagonist use.
• Significant opioid depression occurring in the dental office may require relocation of the patient to a medical facility for comprehensive management.
• Patients discharged from the office/emergency facility should be carefully observed for the return of opioid-induced depression.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


 Acute or Chronic Rheumatoid Arthritis and Osteoarthritis
Acute or Chronic Rheumatoid Arthritis and Osteoarthritis
 Mild-to-Moderate Hypertension, Angina
Mild-to-Moderate Hypertension, Angina Dosage in Renal Impairment
Dosage in Renal Impairment Endometriosis
Endometriosis Central Precocious Puberty
Central Precocious Puberty Tinea Pedis, Tinea Cruris, Tinea Corporis
Tinea Pedis, Tinea Cruris, Tinea Corporis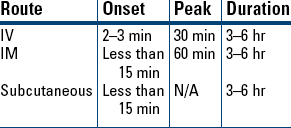
 Analgesia
Analgesia Supplement to Anesthesia
Supplement to Anesthesia Reversal of Opioid Depression
Reversal of Opioid Depression Known or Suspected Opioid Overdose
Known or Suspected Opioid Overdose
 Opioid Toxicity
Opioid Toxicity Postanesthesia Narcotic Reversal
Postanesthesia Narcotic Reversal Neonatal Opioid-Induced Depression
Neonatal Opioid-Induced Depression