Chapter 13
Management of orofacial pain and other co-morbidities in oropharyngeal and nasopharyngeal cancer patients
13.1 Oropharyngeal or Nasopharyngeal Cancer Pain
It is estimated that over 50 million people are partially or totally disabled due to pain and it is a common symptom of patients with cancer.1 Pain in cancer accounts for 30–40% of the main complaints of cancer patients and is of multifactorial etiology. Pain may be a presenting symptom of primary tumors, metastatic disease, systemic cancer, or distant nonmetastasized cancer. When patients have cancer, pain is an extremely common co-morbidity afflicting 65–85% of this population. In approximately 20–40% of cancer patients, pain is described as an agonizing severe pain.2 It is estimated that, worldwide, 3 million people require treatment for cancer pain every year. Although pain is only one of the innumerable symptoms of cancer, it affects physical functions, has emotional impact, and affects the quality of life.3 In head and neck cancer, pain affects the oral functions and is the complaint in about 58% of the patients awaiting treatment, and in 30% of treated patients.4
13.2 Pain Prevalence in Cancer Patients
The various factors that influence cancer pain prevalence include primary tumor type, stage of tumor, and proximity of tumor to neural tissue. Patients with cancer often have multiple causes of pain and multiple sites of pain.5–9 Based on a variety of survey data, up to one-third of patients had more than one pain and 81% of patients reported two or more distinct pain complaints; 34% reported three pains. A 2002 National Institutes of Health (NIH) State-of-the-Science panel found that pain is one of the most common side effects of cancer and cancer treatments.10
A 2007 study examined the prevalence of pain in cancer patients by targeting hospitalized cancer patients in Norway.11 They surveyed 453 individuals and found that 52% were having cancer-related pain with a mean pain level of 4 on a 10-point scale in spite of their medications. A similar study with similar results was performed by a group of physicians in Italy.12 This group administered a questionnaire to 258 cancer patients hospitalized at the National Cancer Institute of Milan. They found 133 patients (51.5%) had pain. They further reported that 49.6% of these patients had pain because of their cancer surgery and 29.3% had pain because of the tumor mass itself. One study in 2008 conducted a national cross-sectional survey of cancer patients in oncologic wards in Italy and found 901 (34%) of 2655 patients had pain with higher pain levels observed in inpatients and those with bone metastases.13
A study to understand the prognoses and preferences for outcomes and risks of treatments in cancer showed that 50% of adults who die in the hospital experience moderate to severe pain in the last 3 days of life.14 The 10-year experience of a German anesthesiology-based pain service associated with a palliative care program reported on the course of treatment of 2118 patients over a period of 140,478 treatment days.15 In their survey, gastrointestinal and head and neck cancers were the most common types, with the majority of pain (85%) caused by tumor involvement. Pain intensity data were collected throughout the course of treatment. Eighty-two percent of patients had moderate to very severe pain at the beginning of treatment, but only 7% reported pain of such high intensity at the completion of treatment.
13.3 Orofacial Pain as the First Sign of Oropharyngeal and Nasopharyngeal Cancer
Cuffari et al. (2006) examined how often pain was reported to be the first clinical sign of oral cancer by looking at the hospital charts of 1412 patients (1977–1998) with oral cancer (238 female and 1174 male).16 Pain was reported as the initial complaint on average in 19.2% of their sample and even categorized the types of pain experienced by these patients (Table 13.1). Orofacial pain has not only been the initial complaint of primary oral cancer patients but has also been reported to be one of the earliest indicators of recurrent cancer. Wong et al.17 described 12 patients who experienced recurrence of primary head and neck cancers that were preceded by severe orofacial pain. When the pain was reported, the authors described their patients as not demonstrating other evidence of malignant disease despite clinical examination, plain radiography, computed tomography (CT), and even magnetic resonance imaging (MRI) of the area.
Table 13.1 Types of pain reported as the initial symptom in oropharyngeal cancer patients
| Pain type | Percentage of sample |
| Sore throat pain | 37.6% |
| Tongue pain | 14.0% |
| Mouth pain | 12.9% |
| Pain when swallowing | 11.1% |
| Dental pain | 05.9% |
| Earache | 05.9% |
| Pain in the palate | 04.1% |
| Burning mouth | 03.3% |
| Gingival pain | 02.2% |
| Pain when chewing | 01.1% |
| Neck pain | 01.1% |
| Facial pain | 00.7% |
Table derived from Cuffari et al., Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2006;102(1):56–61 (ref. 16).
13.4 Co-Morbidities as a Result of Cancer and Its Therapy
Patients treated by surgical excision, radiotherapy, and chemotherapy for cancers frequently experience other problematic orofacial symptoms as well.18,19 The most substantial chronic oral side effects include pain (neuropathic pain and mucositis), dysfunction (trismus or contractures of the jaw muscles), and oral sensory alterations (numbness and sensory distortions). In addition to these treatment complications, patients with a hematologic cancer who undergo bone marrow transplant therapy and immunosuppression will also demonstrate severe oral and pharyngeal mucositis secondary to graft-versus-host disease (GVHD). Mucositis and GVHD are covered in detail in Chapter 12.
13.4.A Cancer-Related Neuropathic Pain
Cancer pain is often referred to as a mixed mechanism pain, as it rarely presents as a pure neuropathic, visceral, or somatic pain syndrome, but rather a complex syndrome with components of inflammatory, neuropathic, and/or ischemic mechanisms often in multiple sites.20 Neuropathic pain is caused directly by cancer-related pathology (compression or infiltration of nerve tissue) or by diagnostic and therapeutic procedures (surgical procedures, chemotherapy, radiotherapy). Manfredi et al. (2003) examined painful neural lesions in 187 cancer patients with pain, referred to a cancer hospital. Based on medical history, pain descriptors, physical examination, and radiological and electrophysiological studies, the pain was categorized as “neuropathic” in 103 patients.21 The most frequent sites of neurological injury were nerve roots, spinal cord and cauda equina, brachial and lumbosacral plexus, and peripheral nerves. There were no patients with pain due to brain injury. In 93 of these patients, the pain was caused by ongoing neural injury; in 10 patients, the neural injury was old and stable.
Work on cancer-related neuropathic pain (chemotherapy induced, or direct invasion) has identified distinct differences in the signature of neuroreceptor–transmitter alterations and unique damage and disruption to neuronal function, and it may yet reveal differences in initiation and maintenance. This evidence would suggest unique features of cancer-related neuropathy, giving a unique molecular signature, while acknowledging some similarity to non-cancer-related neuropathies.22,23 Although the exact prevalence of neuropathic pain in cancer patients remains unknown, it is predicted that at least 15–20% of patients are likely to suffer from neuropathic pain during the course of the disease, and an even higher proportion at advanced stages of the disease.24
Chemotherapy-induced peripheral neuropathy is a common side effect observed following exposure of patients to the vinca alkaloids, the taxanes, the platinum-derived compounds, suramin, thalidomide, and most recently also associated with bortezomib therapy. Reports on the incidence range widely among various studies anywhere between 10% and 100%.25,26 This neuropathy typically affects mostly the small myelinated and unmyelinated nerve fibers. In a phase I trial, patients receiving paclitaxel (Taxol) developed symptoms of neuropathy as early as 1–3 days following treatment.27 Specific signs and symptoms of chemotherapy-induced peripheral neuropathy with cisplatin, oxaliplatin, and vincristine are listed in Table 13.2. Another nonsurgical example of cancer-treatment-induced neuropathic pain is peripheral neuropathy secondary to chronic GVHD in BMT recipients.28
Table 13.2 Agent-specific signs and symptoms of chemotherapy-induced peripheral neuropathy
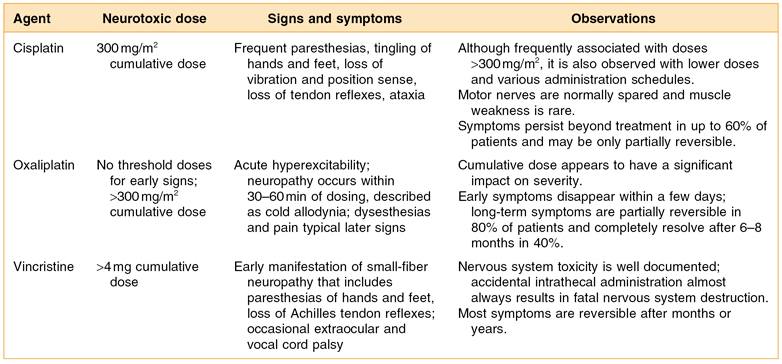
Table derived from from Fine PG, Miaskowski C, Paice JA. Meeting the challenges in cancer pain management. J Support Oncol. 2004 Nov–Dec; 2(6 Suppl 4):5–22.
Neuropathic pain either due to or after cancer surgery has been reported to have a prevalence of 25%.29 Surgical interventions which stretch or transect the nerve have a higher incidence of painful sequelae. Surgical interventions such as mastectomy or debulking tumors often results in deafferentation pain. Postmastectomy patients report a constellation of symptoms, including pain or discomfort in the chest wall, surgical scar, upper arm, and shoulder, which may be suggestive of intercostobrachial nerve damage, and phantom breast sensations.30 Finally, radiation-induced fibrosis can injure peripheral nerves (e.g., fibrosis of brachial plexus) causing chronic neuropathic pain that begins months to years following treatment.31
13.4.B Limited Mouth Opening Secondary to Muscle Spasm and Contracture
Trismus, a tonic contraction of the jaw-closing muscles, has now received broader application in use, including all conditions characterized by the inability to open the mouth adequately. Normal maximal mouth opening ranges from 40 to 60 mm. A mouth opening of less than or equal to 35 mm is a functional cutoff point for trismus in head and neck oncology patients.32 In head and neck carcinoma patients, it is very difficult to discriminate the true cause of trismus. It has been reported as the mechanical obstruction of the mandibular coronoid process and/or condylar process, secondary to local tumor extension into the pterygoid musculature, buccal mucosa, or retromolar area, as well as infratemporal fossa or pterygoid muscle fibrosis in the postirradiation period.33–35 A number of studies have reported trismus in patients with malignant tumors in the head and neck.36–38 Ng and Wei (2006) reported on the prevalence of trismus of the jaw (defined as an interincisal opening less than 25 mm) in 41 patients who had undergone maxillary swing surgery to treat nasopharyngeal carcinoma.39 They found that eight patients (20%) developed postoperative fistulas, and 29 (71%) had severe trismus. Goldstein et al. (1999) suggested that the most decisive factor in whether trismus develops or not is probably the inclusion of the medial pterygoid muscles in the treatment portal during surgery or radiotherapy.40 Inchimura and Tanaka (1993) reported that trismus developed in 21 of 212 patients, of whom 4 showed the symptom at the first presentation and the remaining cases showed the symptom during or after treatment.41 Trismus after irradiation is found in between 27% and 30% of patients with nasopharyngeal carcinoma.42,43
Treatment of surgical or radiation induced persistent postoperative trismus or contracture generally has a low prognosis. The primary approach when a patient exhibits persistent limited mouth opening is (1) stretching under sedation to distinguish trismus from contracture and (2) weekly office and daily home use of a mechanical jaw-stretching device.44 The Therabite® (Fig. 13.1) is one such device that is available on the market today (http://www.atosmedical.com/Products/Mouth_Jaw/The_TheraBite_System.aspx).
Figure 13.1 (A, B) Using Therabite® to improve limited mouth opening.
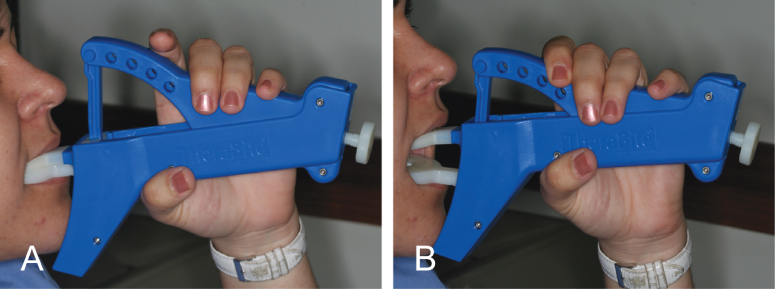
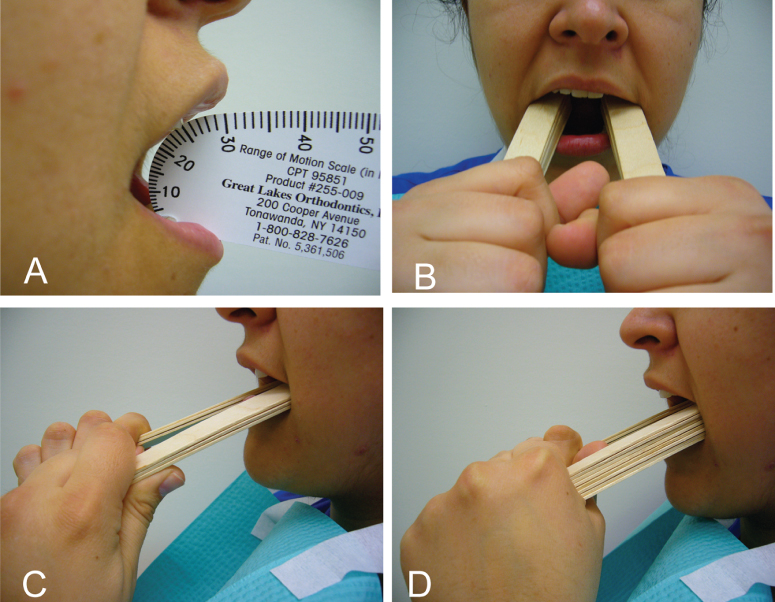
A low-cost alternative to the Therabite includes the use of a stack of tongue blades to increase the size of mandibular opening (Fig. 13.2). Buchbinder et al. (1993) compared Therabite with tongue-blade therapy and unassisted jaw opening exercises in postirradiated patients and found that a sustained increase in mouth opening was achievable only with the Therabite 10 weeks following initiation of therapy.45 The gain in mouth opening for the Therabite group was 6.6 mm greater than the tongue-blade therapy group and 9.2 mm greater for the unassisted exercise group. Stretching exercises or devices need to be implemented early and aggressively in the treatment period to maintain maximum opening and jaw mobility.46 In another study, the use of Therabite was shown to improve the maximal interincisal opening, in postsurgical trismus patients, by an average of 10 mm.47 Patient compliance and perseverance are critical factors for successful treatment outcome despite the choice of therapy.
Figure 13.2 (A) Limited mouth opening of 25 mm. (B, C, D) Using stacks of three to eight tongue blades as a home-based exercise protocol to improve limited mouth opening.
Surgical measures such as coronoidectomy have been reported to be efficient in improving trismus that is not responsive to physical therapy. Bhrany et al. (2007) reported on 18 patients with radiation- or surgery-induced trismus who underwent either unilateral (n = 3) or bilateral (n = 15) coronoidectomies to improve mouth opening.48 These patients had tried and not benefitted from physical therapy in the form of tongue blades or Therabite. Overall, the mean increase in interincisal difference immediately postprocedure was 27 mm, decreasing to 22.2 mm at 6 months postprocedure. Patients followed for 1 year maintained the effect of coronoidectomy, having a mean improvement in interincisal distance of 22.7 and 21.8 mm at 6 months and 1 year, respectively. When the cancer disease or treatment is causing spastic reaction in the jaw-closing muscles, botulinum toxin (BoNT) injection into the involved muscles provides the needed spasticity control.49 The use of BoNT for the orofacial musculature is covered in detail in Chapter 11.
Finally, a systematic review of the literature was conducted by Dijkstra et al. (2004) to identify criteria for trismus in head and neck cancer, risk factors, and the interventions to treat trismus.50 Nine different criteria for trismus were found without justification for these criteria. Radiotherapy (follow-up, 6–12 months) involving the structures of the temporomandibular joint and or pterygoid muscles was found to reduce mouth opening by 18%. Exercises using a Therabite device or tongue blades increased mouth opening significantly. Microcurrent electrotherapy and pentoxifylline were also shown to increase mouth opening significantly.
13.4.C Oral Neurosensory Alterations
Somatosensory abnormalities that interfere with speech, mastication, swallowing, voice quality and resonance, and intraoral sensations are not uncommon in patients who have undergone treatment for oral and nasopharyngeal cancer. Due to these complications, many head and neck cancer teams include a speech and swallowing rehabilitation protocol as part of their postcancer treatment procedures.
Speech, Masticatory, and Swallowing Deficits
It is very well established in the medical literature as to how common chewing, speech, and swallowing problems are after head and neck cancer therapy. Borggreven et al. (2005) analyzed speech outcome for patients with advanced oral or oropharyngeal cancer treated with reconstructive surgery and adjuvant radiotherapy.51 Speech tests (communicative suitability, intelligibility, articulation, nasality, and consonant errors) were performed in a control group and in patients before treatment (n = 76), and 6 months (n = 51) and 12 months (n = 42) after treatment. Speech tests were significantly worse for patients before and after treatment compared with the controls. Speech did not improve between 6 and 12 months. After treatment, patients with T3–4 tumors showed a significantly worse score for communicative suitability, intelligibility, and articulation than patients with T2 tumors. Markkanen-Leppänen et al. (2006) prospectively examined the articulatory proficiency of “r” and “s” sounds, voice quality and resonance, speech intelligibility, and intraoral sensation were before operation and at four time points during a 1-year follow-up after microvascular transfer.52 Forty-one patients with a large oral or oropharyngeal carcinoma undergoing tumor resection and free-flap reconstruction usually combined with radiotherapy participated in the study. Articulation, voice, and resonance were investigated both live and from recorded speech samples by two trained linguistic examiners. The patients completed a self-rating of their speech intelligibility and were assessed for anterior intraoral surface sensation by means of two-point moving discrimination. Misarticulations of “r” and “s” increased significantly after the therapy. Voice quality and resonance remained essentially normal. Speech intelligibility deteriorated significantly. Intraoral sensation decreased postoperatively but was not related to speech outcome. Sensate flaps did not prove to be superior in relation to speech tasks. The authors advocated a multidisciplinary approach in assessment of speech outcome after cancer surgery. Speech therapy was strongly recommended, even in the absence of a gross articulatory handicap.
McConnel et al. (1998) conducted a multi-institutional prospective study of speech and swallowing function before and after soft-tissue reconstruction of the oral cavity and oropharynx, and compared three methods of reconstruction with respect to speech and swallowing function: (1) primary closure, (2) distal myocutaneous flap, and (3) microvascular free flap53; 284 patients treated at the four leading head and neck cancer institutions were matched for the location of the oral cavity or oropharyngeal defect and the percentage of oral tongue and tongue base resection. The patients underwent speech and swallowing evaluation preoperatively and 3 months after healing. This evaluation included videofluoroscopic studies of swallowing and tests of speech intelligibility and sentence articulation. Videofluoroscopy provided measures of swallowing efficiency and bolus movement. Liquid and paste consistencies were used in evaluating swallowing function. They found that patients who had primary closure were more efficient at swallowing liquids, had less pharyngeal residue, a longer oral transit time with paste, and higher conversational intelligibility than patients who underwent reconstruction with a distal flap. Compared with patients who underwent reconstruction with a free flap, those who had primary closure had more efficient swallowing of liquids, less pharyngeal residue, and shorter pharyngeal delay times with paste. No difference in the speech and swallowing function existed between patients treated with distal myocutaneous flaps and those treated with microvascular free flaps. The authors found that the use of primary closure resulted in equal or better function than the use of flap reconstruction in patients with a comparable locus of resection and percentage of oral tongue and tongue base resection.
In 2007, Borggreven et al. conducted another study to assess the swallowing outcome in advanced oral or oropharyngeal cancer patients treated with microvascular reconstructive surgery and adjuvant radiotherapy.54 Postoperative videofluoroscopic swallowing studies (VFSS) and scintigraphy tests were performed at 6 and 12 months in 80 patients. Swallowing parameters such as the oropharyngeal swallow efficiency and the Penetration/Aspiration Scale were analyzed and impaired swallowing status was found at 6 months, which remained stationary at 12 months. Larger tumors (T3–T4 vs. T2) and resections of the base of tongue and soft palate combined (vs. defects of other dynamic structures) were associated with most profound swallowing problems (p < 0.05). In a similar study, Zuydam et al. (2000) reported that oropharyngeal cancer patients who had undergone surgical resection had swallowing disorders. The disorders were related to the extent of the resection and the consistency of the bolus.55 Those with involvement of a quarter of the tongue base or more generally had greater impairment, and radiotherapy tended to exacerbate these problems. Aspiration was a major problem in these patients. Interestingly, compensatory procedures and therapy techniques such as chin tuck and supraglottic swallow were effective in 50% of patients who aspirated, and tended to be more effective between the 1-month and 6-month follow-up in patients with smaller resections.
The previously cited paper by Markkanen-Leppänen et al. (2006) also prospectively assessed the swallowing and intraoral sensation outcomes after microvascular free-flap reconstruction in 41 patients with a large oral or oropharyngeal carcinoma who had undergone free-flap surgery, usually combined with radiotherapy. The patients completed modified barium swallow, self-rating of swallowing, and two-point moving discrimination preoperatively and at four time points during a 12-month follow-up period. A plain chest X-ray was done 1 year after operation. Intraoral sensation deteriorated and swallowing was impaired with respect to an objective and subjective measure after therapy. Rates for nonsilent and silent aspiration increased during the follow-up and the swallowing outcome was not related to sensation. One year after surgery, 86% of the patients ate regular masticated or soft food. The authors concluded that microvascular transfers offer a reasonable option for oral reconstruction. Swallowing problems should be routinely sought and patients rehabilitated during a sufficiently long follow-up with videofluorography regardless of the patient’s perception of swallowing. In conclusion, head and neck cancer patients who had microvascular free flap reconstruction or primary closure seem to have better outcomes with respect to speech and swallowing defects in comparison to those who had a distal flap reconstruction only.
Masticatory, speech, and swallowing problems are not inherent to only head and neck cancer patients. Gurney et al. (2006) examined the long-term effects of hematopoietic stem-cell transplantation therapy on 235 childhood cancer survivors.56 The study was unique in that it used 705 noncancer siblings as their control group. All participants completed a survey with questions on post-transplant impairments, and the median length of follow-up was 11 years. Interestingly, persistent pain was reported by 21% of survivors and they were also 7.7 times more likely to report chewing or swallowing problems.
Intraoral Sensory Alterations
Bodin et al. (1999) tested oral sensory discrimination using a hole size identification test in 31 patients with a diagnosed malignant tumor of the oral cavity or pharynx.57 The testing was performed four times (before treatment, after radiotherapy, and 6 months and 1 year after surgical treatment). The study included a control group of healthy individuals of the same age who were tested two times at a 2-month interval. The results showed the sensory discrimination ability in the oral cancer patients was not diminished after radiotherapy, but it was after cancer surgery and this change was still present after 1 year. In contrast, the pharyngeal cancer group did not have a change in their oral sensory discrimination after radiotherapy or surgery. The authors concluded that “cancer surgery of the oral structures causes a persistent loss of sensory discrimination.” They speculated that this might contribute to the frequently seen mastication and swallowing difficulties exhibited by oral cancer surgery patients. Also, the patients’ capabilities of shape recognition had deteriorated significantly with no difference between the oral cancer group and the pharyngeal cancer group and the nonoperated side did not compensate for the operated side.58 Bodin et al. (2004) conducted a similar study on 27 patients and 20 controls with oral cancer who had undergone only radiation therapy.59 A delayed deterioration of oral sensation was revealed on the nontumor side 6 months after radiotherapy and there was no recovery in this deterioration even 1 year post-treatment. Patients who had undergone mandibular resection for benign tumors such as ameloblastomas suffered some degree of neurosensory deficit, but some recovery was seen especially in patients younger than 16 years.60
Surgeons have been performing reconstructive surgery to repair surgical defects in the head and neck region using flaps for several decades. Interestingly, in recent years, microvascular reconstructive surgery with anastomosis of nerves from the flap to the severed nerves at the surgical site has been shown to decrease neurosensory deficits and improve sensation by at least 50%. Sensate flaps have been shown to be more superior to nonsensate flaps. Boyd et al. (1994) showed that patients who received sensate radial forearm flaps in which the lateral antebrachial cutaneous nerve was anastomosed to the (divided) lingual nerve had greater two-point discrimination and pressure sensitivity compared with the ones who received noninnervated radial forearm flaps.61 One rather interesting finding in this study was that patients who received pectoralis flaps had lesser sensory re-innervation compared with those who received either innervated or noninnervated radial forearm flaps. This difference in sensory perception apparently is due to the fact that radial forearm flaps have greater density of free-nerve endings compared with pectoralis flaps. Another interesting finding in this study was that the sensory discrimination in the forearm flaps was greater even though that degree of discrimination is not normally present in the forearm. The explanation for this finding is that the flap which is anastomosed with the lingual nerve is now represented by a larger area (for the tongue) in the sensory cortex compared to the area for the forearm. Kimata et al. (1999) conducted a study comparing differences in sensation between innervated and noninnervated thigh flaps and rectus abdominus flaps.62 As in the earlier study, the innervated flaps had a greater degree of sensation and the degree of sensory recovery of innervated thigh flaps was significantly greater than that of innervated rectus abdominus flaps. Similarly, greater sensory recovery has been reported in fasciocutaneous radial forearm flaps compared with jejunal flaps.63
Finally, mental neuropathy may be the first manifestation of systemic cancer, a symptom of spread of an established tumor, or a sign of infiltration in an intraoral lesion. It is characterized by the presence of a sensory defect in the form of paresthesias or dysesthesias in the territory innervated by the mental nerve and is indicative of a very poor patient prognosis. Sanchis et al. (2008) studied 22 cancer patients with chin paresthesia.64 The patients were divided into two groups. Group 1 comprised patients (n = 11) with chin paresthesia who had a primary tumor in some other region at a distance from the oral cavity or maxillofacial zone. Group 2 (n = 11) in turn comprised patients with primary malignancies of the oral and/or maxillofacial territory and who likewise presented with chin paresthesia. Data were collected relating to patient age, gender, primary intraoral lesion (location, size, histologic diagnosis), primary systemic tumor, and mean patient survival. In group 1, the mean survival after the diagnosis of chin paresthesia was 14.8 ± 16.5 months and only 1 patient was still alive after 9 months. Group 2 consisted of 11 patients with oral squamous cell carcinoma, with the exception of 1 case of fibrosarcoma. In this group the mean survival of the 8 patients who died was 28.2−29.6 months. Three patients survived for a mean of 17 months. The authors concluded that chin paresthesia is a very important prognostic symptom determining the degree of infiltration of intraoral lesions, and in some cases it may be indicative of the existence of a primary tumor (identified or otherwise), with poor short-term survival given that 81.9% of the patients studied (18 cases) had died before a mean of 20 months. Although mean survival was shorter (14.8 months) among the patients in group 1 than in group 2 (28.2 months), the difference was not found to be statistically significant.
13.5 Challenges in Cancer Pain Management
Barriers to pain management include issues related to clinicians, patients, and the health system. The traditional model of care is focused on disease-specific treatments. If these treatments fail, the focus shifts to palliation. The most general and common physician-related barriers to cancer pain management are concerns about side effects to opioids, prescription of inefficient doses of opioids, and very poor prescription for the treatment of side effects from opioids.65 With regard to the use of analgesics for cancer pain in the United States, a survey reported that 86% of physicians felt the majority of patients with cancer pain were undermedicated. Only 51% believed pain control in their own practice setting was good or very good; 31% would wait until the patient’s prognosis was 6 months or less before they would start maximal analgesia. Adjuvants and prophylactic side-effect management were infrequently used in the treatment plan. Concerns about side-effect management and tolerance were reported as limiting analgesic prescribing. Poor pain assessment was rated by 76% of physicians as the single most important barrier to adequate pain management. Other barriers included patient reluctance to report pain and patient reluctance to take analgesics (both 62%) as well as physician reluctance to prescribe opioids (61%).66 A study of 4000 elderly nursing home residents with cancer revealed that 24%, 29%, and 38% of those over age 85 years, 75–84 years, and 65–74 years, respectively, reported daily pain.67 Twenty-six percent in daily pain did not receive any medication. Those older than 85 years who reported pain were most likely to receive no analgesic. There is a need for educational programs in cancer pain targeted toward healthcare practitioners to better understand these barriers and address them effectively.
13.6 Management of Cancer Pain
Regardless of whether the pain is neuropathic, nociceptive, cancer induced, or cancer-treatment induced, if it is severe, opioids are widely utilized for pain relief. For mild-to-moderate pain, nonopioid analgesics and other adjunctive medications are used per the World Health Organization (WHO) recommendations for cancer pain management.
13.6.A Pharmacologic Management: NSAIDs and Nonopioid Analgesics
The use of nonsteroidal anti-inflammatory drugs (NSAIDs) and nonopioid analgesics such as acetaminophen is common in cancer pain management. In fact, pain specialists often undertake combination therapy with multiple analgesics, including two or more analgesics, during the treatment of severe, refractory pain. The use of NSAIDs along with opioids in cancer patients reduces the need for an opioid dose escalation or allows the use of lower doses. Their use is associated with a more intense gastric discomfort, but results in less opioid-related constipation.68 In cancer patients, the use of NSAIDs may delay the development of opioid tolerance although central toxicity may be observed with NSAIDs. These adverse effects may interfere with optimal patient function, and limit the quality of residual life. Diclofenac sodium and ketorolac are popular NSAIDs for cancer pain and are often used with opioids. Diclofenac does not modify morphine or methadone pharmacokinetics in cancer patients, which indicates that its analgesic effect is independent of any modification of the opioid. Ketorolac has been reported to be effective for malignant bone pain secondary to metastatic invasion.69 The addition of NSAIDs is particularly useful for patients experiencing opioid toxicity upon escalating the opioid dose.70
History of peptic ulcer disease, advanced age (>60 years of age), female gender, and concurrent corticosteroid therapy should be considered before NSAID administration to prevent upper gastrointestinal tract bleed/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


