Chapter 12
Treatment for oral mucositis and noninfectious, non-neoplastic oral ulcerations
12.1 Introduction
This chapter focuses on two mucosal disease problems that cause significant pain and discomfort. First we review oral mucositis (OM) induced by radiotherapy and/or chemotherapy. Second, we review multiple non-neoplastic, noninfectious oral ulcerative (OU) conditions. As mentioned, both conditions cause substantial pain, and patients with either condition need help with pain management. Fortunately, for OM there are recent evidence-based guidelines for treatment that have been endorsed by the Multinational Association of Supportive Care in Cancer (MASCC) and the International Society for Oral Oncology (ISOO).1,2 Where we could find them, Cochrane-based reviews on these treatment methods have been included.3,4 With regard to the subgroup of non-neoplastic and noninfectious oral ulcerative (OU) conditions, we discuss the palliative and immunosuppressive management methods that are common for almost all of these conditions. Where a specific oral ulcerative condition has a unique and evidence-based treatment method, this is also reviewed.
12.1.A Oral Mucositis
Oral mucositis is a condition that is essentially a side effect of cancer therapy, namely, radiotherapy and chemotherapy. These treatments cause a painful, erythematous mucosa that transforms into even more painful ulcerations.5 The specific features of OM are provided next.
Clinical Presentation
This condition is a painful and often dose-limiting complication of radiotherapy (RT) and combined chemoradiotherapy (CRT) to the head and neck. It also develops in association with standard chemotherapy (CT) protocols used for cancer therapy as well as the high-dose conditioning regimens used with hematopoietic stem cell transplantation (HSCT). The healing period is usually 2–4 weeks after cessation of either therapy. OM along with pharyngeal and other alimentary tract mucositic inflammation can lead to significant complications, including dysphagia, malnutrition, electrolyte imbalance, systemic infection, and even death. If the patient is not already hospitalized, it is not uncommon for a severe OM to force hospitalization and untoward treatment modifications and interruptions.
Location
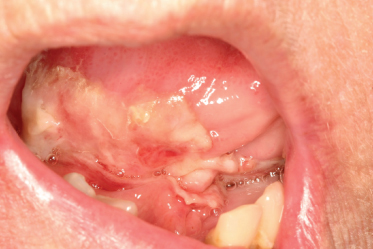
For chemotherapy-induced mucositis, the nonkeratinized mucosa of the oral cavity is typically affected (Figs. 12.1 and 12.2). The keratinized mucosa is generally spared with chemotherapy-induced OM, but may be affected following radiotherapy.
Figure 12.1 Clinical presentation of radiation-induced oral mucositis.
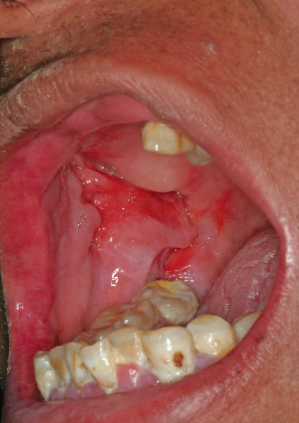
Figure 12.2 Clinical presentation of chemotherapy-induced oral mucositis.
(Photograph courtesy of Nathaniel S. Treister, DMD, DMSc, Division of Oral Medicine and Dentistry, Brigham and Women’s Hospital.)
Grading the Severity of Oral Mucositis
Treatment of secondary OM is largely governed by the severity of the problem (e.g., more severe problem dictates a more aggressive treatment approach) so it is essential to rate OM. Several clinical measurement scales of OM have been used by clinicians and researchers. The World Health Organization (WHO) scale is preferred by many clinicians for its simplicity and ease of use (Table 12.1). This scale measures clinical signs and symptoms as well as functional ability, mainly to eat and drink. The other commonly used scales are the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0,6 the Oral Mucositis Assessment Scale (OMAS),7 and the Eastern Cooperative Oncology Group (ECOG) common toxicity criteria.8
Table 12.1 World Health Organization scale for oral mucositis
| Grade 0 | No oral mucositis |
| Grade 1 | Erythema and soreness |
| Grade 2 | Ulcers, able to eat solids |
| Grade 3 | Ulcers, requires liquid diet |
| Grade 4 | Ulcers, alimentation not possible |
Etiology
The frequency and severity of OM varies with different modalities of oncologic treatment regimens.9–11 As a general rule, OM lesions typically occur 1–2 weeks following chemotherapy or after radiotherapy doses greater than 30 Gy (gray, a unit of absorbed radiation).
12.1.B Oral Mucositis Epidemiology
The type of antineoplastic treatment logically dictates the number and severity of patients who have OM. In a comprehensive analysis of more than 400 studies, Sonis et al. noted that mild mucositis problems (Grades 1 and 2 according to the WHO scale) were not reported consistently in many studies; however, when grades 3 and 4 were combined, data on the incidence of mucositis was determined.12 This study examined which treatment regimes were more likely to cause mucositis and they reported that when 5-fluorouracil (5-FU) was added to a chemotherapy regimen the rate of Grade 3–4 OM was greater than 15%. Use of new agents, such as imatinib and taxane- and platinum-based regimens, was associated with a lower incidence of oral and gastrointestinal mucositis.5,12 In contrast, radiation therapy to the head and neck results in Grade 3–4 OM in at least 50% of patients; depending on the tumor site, it may induce OM in 100% of patients. If a patient is undergoing HSCT with a total body irradiation containing conditioning regimen, the rate of mucositis was around 60% whereas the incidence rate declined to 30–50% with chemotherapy alone.12 Recently (2008), the European Blood and Marrow Transplantation Mucositis Advisory Group reported on OM.13 This study assessed the incidence, duration, and determinants of severe OM (WHO oral toxicity scale grades 3–4) in patients with multiple myeloma (MM) or non-Hodgkin’s lymphoma (NHL) receiving high-dose conditioning chemotherapy before autologous HSCT. They found that of the 109 patients with MM and 88 patients with NHL who were treated, severe OM occurred in 46% of MM patients and 42% of NHL patients respectively. OM incidence rates, however, are at best rough estimates due to the lack of well designed prospective studies.
12.1.C Oral Mucositis Consequences
The burden of OM not only compromises the quality of life (QOL) of the patients but also significantly increases the cost of treatment and hospitalization due to associated pain and secondary infection management, diet and nutritional supplements, and gastrostomy tube placement. In a prospective, multicenter, observational study to evaluate a new QOL instrument, the Oral Mucositis Weekly Questionnaire–Head and Neck Cancer (OMWQ-HN), increasing mouth and throat soreness (MTS) corresponded with a steady decline in function, with the greatest impact on eating, swallowing, and drinking.14 In a retrospective study assessing the in-hospital complications of autologous HSCT in multiple myeloma and lymphoma patients, incident infectious complications, stomatitis, and the use of total parenteral nutrition (i.e., providing nutrition via an intravenous line) increased the mean cost of hospitalization by more than threefold.15 Similar findings were reported in a retrospective cohort of 204 head and neck (HN) cancer patients undergoing radiotherapy or chemoradiotherapy, analyzed for risk, cost, and clinical and economic outcomes of OM. In this study, a total of 91% of patients developed OM of whom 66% had severe mucositis (Grade 3–4). For both OM groups, a substantial increased cost of care was seen.16
12.1.D Oral Mucositis Pathogenesis
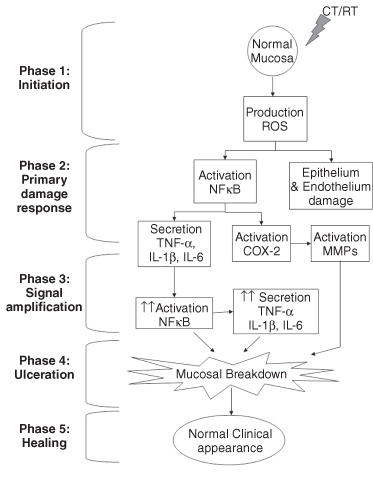
Oral mucositis is the clinical manifestation resulting from a complex sequence of events involving the activation of several intracellular signaling pathways with concomitant molecular changes. It is now known that OM is not a condition exclusively affecting the epithelium, but rather involving all the cellular components of the mucosa. With the cumulative knowledge gained by molecular research over the last decade or so, the proposed mechanisms of OM have evolved into a five-phase (or stage) model.17 Understanding this model may help researchers develop more targeted molecular therapies in the future:
- The initiation phase At the cellular and molecular level, immediately after administration of chemotherapy (CT) or radiation therapy (RT), the direct DNA damage caused by these treatment modalities leads to the production of reactive oxygen species (ROS), which results in death of cells in the basal layer and submucosal cells; collectively these events are referred to as the initiation phase.
- The primary damage response phase This second phase, the primary damage response phase, is characterized by the production of pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-1β, and activation of signaling pathways, such as the ceramide pathway, mediated in large part by the transcription factor nuclear factor kappa B (NF-κB).
- The signal amplification phase The signal amplification phase is characterized by positive feedback via NFκB with further upregulation of TNF-α, IL-6, IL-1β, cyclooxygenase-2 (COX-2), and other pro-inflammatory mediators, amplifying the damage caused to the tissues by increased inflammation and apoptosis.
- The ulceration phase The ulceration phase follows when there is clinically evident breakdown of the mucosa. Pain is greatest during this phase, and secondary bacterial colonization of the ulcerated mucosa can further contribute by triggering more production of pro-inflammatory cytokines.
- The healing phase The healing phase is defined by restoration of the integrity of the mucosal tissues following termination of CT or RT treatment. The mechanisms involved here are yet to be completely elucidated (Fig. 12.3).18,19
Figure 12.3 Summary of the main cellular and molecular events in the five-stage model of the pathogenesis of oral mucositis.
(Adapted from Sonis ST, et al., Cancer, 2004;100(9 Suppl):1995–2025; Sonis ST, Oncol, 2007; 5(9 Suppl 4):3–11.)12,19
Cytokines and Nuclear Factor
Due to the relevance of NF-κB and pro-inflammatory cytokines in the pathogenesis of OM, a more detailed review of its role on this condition is warranted.20–22 It is important to highlight that NFκB is an important transcription factor responsible for the activation of several genes involved in inflammatory processes. NF-κB is activated in response to pathogens and pro-inflammatory cytokines and by radiotherapy and chemotherapeutic agents. In addition, an increase in the expression of COX-2, which is regulated by NF-κB, is also observed, providing further evidence for the role of NF-κB and its target genes on the pathogenesis of OM.23 In regards to the role of TNF-α and IL-1β in OM, the most convincing recent data has come from hamster studies. Hamsters that have received radiation and developed OM have an increased epithelial expression of TNF-α and IL-1β at day 15 postradiation. In this study the higher levels of these cytokines had a positive correlation with the severity of OM.24 Similarly, in rats that had OM induced by treatment with either 5-FU or methotrexate (MTX) there was an increased expression of NFκB, TNF-α, IL-1β, and IL-6 in the involved oral mucosa.25 Agents that block TNF-α, such as pentoxifylline (PTX) and thalidomide (TLD), have been shown to inhibit cytokine synthesis in hamsters, which in turn resulted in decreased OM.26 However, pentoxifylline has been used in clinical trials with no remarkable success for prevention of OM. The MASCC/ISOO panel does not recommend the use of pentoxifylline to prevent OM in patients undergoing HSCT, with similar observation from Cochrane review.27,28
12.2 Oral Mucositis Management
The management of OM is largely palliative, consisting mainly of pain relief, nutritional support, and improved quality of life. With regard to prevention of OM, several pharmacologic interventions have been reported as promising; unfortunately, the majority of studies conducted have not been well designed and are not randomized, placebo-controlled trials. Wherever possible, our recommendations are based on those put forth and endorsed by MASCC and the ISOO and are supplemented with Cochrane reviews whenever applicable.
12.2.A Basic Oral Hygiene, Diet, and Nutrition
All patients who are scheduled to undergo radiotherapy or chemotherapy regimes that have a high likelihood of OM development need to have a careful and detailed review of their oral hygiene practice. In addition they need to understand how their diet must change and how proper nutrition might affect the course of their healing reaction.
Basic Oral Hygiene
Maintenance of oral hygiene is essential in patients with OM to prevent secondary infection and sepsis, to reduce pain, and to improve function. Preventative care may reduce the risk of OM in children.29–31 It is generally agreed that good oral care is an important adjunct to the prevention, severity, and management of oral mucositis, but it is rarely stressed in clinical practice.32 Patients, routinely, are not taught how to care for their mouths, and the nursing practice of assessing for oral complications and educating families on the importance of oral hygiene is often overlooked.33,34 Additionally, oral hygiene greatly varies across and even within clinical institutions. One survey that looked at 92 transplant centers saw few similarities regarding the management of oral care and found that oral care procedures were based on tradition or subjective evaluation rather than evidence-based practice.35,36
Good oral hygiene is the only intervention that has demonstrated a clear benefit in the prevention or treatment of mucositis in children.37 Tooth brushing, flossing, rinsing with a bland agent, such as sterile water, and using mouth moisturizers prevents infections of the oral soft tissue and helps alleviate pain and bleeding.1,38 Oral hygiene should be performed regardless of hematologic status, myelosuppression, and/or thrombocytopenia.39 It is reasonable to continue flossing without traumatizing tissues during immunosuppressive periods and throughout therapy, but dental floss should only be allowed if the patient is properly trained. Though the literature varies regarding the implementation of “basic oral hygiene,” most agree that tooth brushing is the best way to keep the mouth clean, preferable to oral sponges.40,41 Oral sponges or toothettes should only be used when the patient cannot tolerate a regular toothbrush. Tooth brushing should be encouraged two to three times a day, brushing should last at least 2 minutes, and the brushes should be air-dried between uses.42 It is important to note that the toothbrush itself can become colonized with bacterial organisms, stressing the importance of replacing toothbrushes often, such as after each febrile illness and/or cycle of chemotherapy, to prevent excessive accumulation of oral bacteria.43
- The MASCC/ISOO panel recommends regular, systematic, oral care hygiene with brushing (with a soft-bristled toothbrush which should be replaced regularly), flossing, bland rinses, and moisturizers using a standardized oral care protocol for all OM patients.
- The panel notes that an interdisciplinary approach to oral care involving physician, dentist, nurse, dental hygienist, dietician, pharmacist, and others as relevant will be essential for providing comprehensive supportive care for OM patients.1
Diet and Nutrition
Intake of regular diet and maintaining adequate nutrition become very difficult in OM patients. A liquid or soft diet that does not require chewing is recommended. General guidelines include the following: using topical analgesics prior to eating; the intake of small pieces of food, avoiding highly acidic, salty, bitter, and spicy foods; using a straw with liquids; use of nutritional supplements; and nonalcohol mouthwash following meals.44 A gastrotomy tube (enteral nutrition) or a Hickman line (parenteral nutrition) may be placed in some patients in anticipation of severe OM.27
12.2.B Prevention of Secondary Infections
Because incipient dental disease can rapidly progress in a mouth that is not cleaned daily, correction of pre-existing oral conditions prior to the initiation of chemotherapy and subsequent aggressive mouth care reduce the incidence and severity of mucositis.45 Standard care should therefore include dental evaluation and correction of any periodontal disease before beginning chemotherapy. Patients should also receive instruction on mouth care and be encouraged to adhere to an aggressive oral hygiene regimen throughout the treatment period. Maintenance of oral hygiene becomes extremely difficult, thus creating an ideal situation for opportunistic oral and potentially systemic infections. Although routine or prophylactic use of antibacterial,46 antiviral,47 or antifungal48 treatment is not effective in preventing OM, as these microorganisms are not believed to play a role in its pathogenesis, their use in the treatment of a secondary, concurrent infection is appropriate.49
Chlorhexidine
Chlorhexidine rinses at a 0.12% concentration have been widely used in dentistry as an antibacterial mouthrinse, especially in patients with periodontal disease who cannot clean their mouths effectively. Chlorhexidine mouthrinses are used as part of oral decontamination in OM patients in some centers but would typically be discontinued with severe OM onset since the alcohol in the mouthwash would be too irritating. As a preventative agent, chlorhexidine is ineffective in preventing or treating OM.50,51 The Cochrane review concluded that there is insufficient evidence to support or refute that chlorhexidine is more or less effective than placebo or no treatment in prevention of OM.3
- The MASCC/ISOO panel has recommended not using chlorhexidine to prevent OM in patients undergoing radiotherapy for solid tumors of the head and neck or to treat established OM.1
On a practical clinical note, it should be remembered that if mucositis develops, chlorhexidine rinses should be discontinued if irritating, and alcohol-free formulations should be used instead.52
12.2.C Medications for Pain Control in Oral Mucositis
Pain is mostly based on severity and extent of lesions and unfortunately is the hallmark symptom of OM.53 The physical and emotional distress due to the cancer and its treatment can lead to more subjective increase in pain in many patients and is explained by the biopsychosocial model of pain.54,55 The subjective and objective assessment of pain and its impact on function is critical for appropriate management and interventions. The need for proper subjective and objective pain assessment in terms of function and need for medications has been reported.56,57 Management includes systemic analgesics (mainly opioids) and local palliative measures such as topical anesthetics, analgesics, and mucosal protective agents.
Systemic Analgesics
Morphine is the opioid that has been used primarily for management of OM pain either as patient-controlled analgesia (PCA) or hospital infusions.58 PCA has been shown to require less total opioid use for the management of OM pain compared with continuous hospital infusion with equivalent levels of pain control.59,60 Interestingly, another mode of drug delivery called pharmacokinetically based, patient-controlled analgesia (PKPCA), where patients adjust the rate of continuous morphine infusion to increase or decrease their plasma morphine concentration, was shown to provide more pain relief than conventional bolus dose PCA without a significant increase in side effects.61
- The MASCC/ISOO panel recommends PCA with morphine as the treatment of choice for OM pain in patients undergoing HSCT.
There may be a role for tricyclic antidepressants (TCAs) and fentanyl transdermal therapeutic system (FTTS) in the management of OM pain but further studies are needed.62,63 Chapter 4 covers the proper use of opioids for malignant and nonmalignant pain.
Topical Anesthetics and Analgesics
Topical anesthetics such as 2% viscous lidocaine and “magic” mouthwash (a term that refers to different formulations used as mouthwash and consisting of a mixture of ingredients). While the formulations vary, often magic mouthwash is made of equal parts viscous lidocaine (2%), liquid diphenhydramine, and a mucosal coating agent (e.g., Maalox® or kaopectate). The typical approach is to have the pharmacist mix these agents together, dispense them with a 15-mL dosing cap and instruct the patient to keep this liquid cool (stored in the refrigerator at home). The patient is instructed to rinse with 15 mL up to four times a day. Specifically, the patient should swish it in the mouth for 30 seconds and then spit out. Compared with saline rinses, topical lidocaine is much more effective at reducing pain secondary to OM. Sometimes anti-inflammatory agents are not adequate. For example, a randomized study with a small group of 26 OM patients being treated with concomitant chemoradiotherapy for head and neck cancer compared the efficacy of morphine mouthrinse versus a magic mouthwash (i.e., equal parts lidocaine, diphenhydramine, and magnesium aluminum hydroxide).64 The authors reported that the duration of severe pain and intensity of oral pain were found to be significantly lower in the morphine group than the magic mouthwash group. A recently published randomized double-blinded crossover study showed significant pain relief with topical morphine compared with placebo and the pain relief lasted for almost 2 hours with topical morphine solution.65 Additional information on topical anesthetics for oral pain can be found in Chapter 5.
12.2.D Mucosal Protective Agents
Several agents or formulations have been used and studied as a protective mucosal barrier to aid in patient comfort during OM.66 Of these, sucralfate, Gelclair® (Helsinn Healthcare SA, Lugano, Switzerland), and Caphosol® (Cytogen Corporation, Princeton, NJ) are briefly discussed here.
Sucralfate
Sucralfate, a basic aluminum salt of sucrose sulphate has been used for both prevention and treatment for OM. However, studies have not shown much benefit.67,68 The Cochrane review found no statistically significant differences, concluding that there is insufficient evidence to support or refute that sucralfate is more or less effective than placebo.
- The MASCC/ISOO panel does not recommend the use of oral sucralfate for reduction of side effects of radiotherapy.
Gelclair
This agent comes as an oral gel containing mainly polyvinylpyrrolidone and sodium hyaluronate that forms an adherent barrier over the oral mucosa. Its protective barrier forming abilities seem to provide transient comfort to OM patients.69 A recently reported randomized clinical trial comparing Gelclair against a mixture of sucralfate and Mucaine® (which mainly contains oxethazaine in alumina gel of aluminum hydroxide and magnesium hydroxide; Wyeth-Ayerst Laboratories) in 20 patients with radiotherapy-induced OM found no significant difference between the two. Both agents provided good short-term pain control but relief did not last for the full 24-hour assessment period in this study. Further randomized, placebo-controlled trials will have to establish the efficacy of this agent.
Caphosol
This agent comes as a mouthrinse and it is a neutral, supersaturated, calcium phosphate (Ca2+/PO43−). Calcium and phosphate downregulate the inflammatory process, blood clotting cascade, and tissue repair and are believed to exert their beneficial effects by diffusing into intercellular spaces in the epithelium and permeating OM lesions. A double-blind, prospective, randomized clinical trial studied the efficacy of Caphosol with fluoride treatments against a standard regimen of fluoride rinsing and placebo tray treatments in 95 patients undergoing HSCT. The authors found a statistically significant decrease in days of OM and duration of pain, besides other parameters.70 While these findings appear to be promising, no additional studies have been published and the result has not been replicated at other centers.
12.2.E Chemopreventative agents to prevent and/or reduce severity of oral mucositis
The idea that you can use a medication before the radiotherapy or chemotherapy to ward off or minimize the severity of OM has received much attention.
Cryotherapy
Cryotherapy has been shown to reduce OM in patients receiving stomatotoxic chemotherapy with a short half-life and doses over short periods of time.71–73 Oral cryotherapy causes local vasoconstriction, reduces blood flow to the oral mucosa thereby decreasing the uptake of 5-FU, and eventually less OM. In cryotherapy, ice chips are typically placed in the mouth 5 minutes before administration of chemotherapy and are replenished over 30 minutes. It should be noted that cryotherapy is not useful in radiation-induced OM as it causes direct mucosal damage in the radiation field.
- The MASCC/ISOO panel recommends that patients receiving bolus 5-FU chemotherapy undergo 30 minutes of oral cryotherapy to prevent OM; it also suggests the use of 20–30 minutes of oral cryotherapy to decrease OM in patients treated with bolus doses of edatrexate and the use of cryotherapy in patients receiving high-dose melphalan as a conditioning agent in HSCT.
Keratinocyte Growth Factors
Keratinocyte growth factor (KGF) is a 28-kDa heparin-binding member of the fibroblast growth factor (FGF) family and specifically binds to the KGF receptor expressed only in epithelial tissues. KGF mediates proliferation and differentiation in a wide variety of epithelial cells, including keratinocytes in stratified squamous epithelia.74 Systemic administration of recombinant human KGF (rHuKGF) has been shown to provide significant epithelial protection in animal models of epithelial–mucosal damage.75 The successful animal studies led to studies in humans with recombinant human KGF (palifermin), which was shown to cause epithelial proliferation as well, especially in patients with OM.76–79 Recombinant human KGF1 (fibroblast growth factor 7 [FGF-7] or palifermin) is a member of the FGF superclass. The cytoprotective effect of palifermin is attributed to several functions: mitogenic effect which results in increased thickness of mucosal epithelium; upregulation of Nrf2, a transcription factor in keratinocytes which upregulates genes encoding reactive oxygen species–scavenging enzymes;80 generates IL-13, an anti-inflammatory cytokine which reduces TNF-α; exerts antiapoptotic effects; and reduces angiogenesis. The US Food and Drug Administration (FDA) approved palifermin (Kepivance®) for patients with hematologic malignancies receiving chemotherapy and radiation therapy and requiring HSCT.81
- The MASCC/ISOO panel recommends the use of keratinocyte growth factor-1 (palifermin) in a dose of 60 µg/kg per day for 3 days prior to conditioning treatment and for 3 days post-transplantation for the prevention of OM in patients with hematologic malignancies who are receiving high-dose chemotherapy and total body irradiation with autologous HSCT.
Another KGF, repifermin (KGF-2) was studied in a Phase I/II randomized, placebo-controlled trial evaluating the safety and clinical effects to reduce OM in 42 patients undergoing autologous HSCT.82 This agent, however, did not demonstrate efficacy.
Granulocyte–Macrophage Colony Stimulating Factor
Granulocyte–macrophage colony stimulating factor (GM-CSF) stimulates cells of the innate immune system in mucosal tissues. Several studies evaluating the usefulness of GM-CSF mouthwash in OM patients found that it did not decrease the frequency and duration of severe OM in patients.83–85 The Cochrane reviewers after evaluating nine trials until 2003 concluded that the comparisons between GM-CSF and placebo or no-treatment groups were not significant and therefore there is insufficient evidence to support or refute the efficacy of GM-CSF.
- The MASCC/ISOO panel suggests that GM-CSF mouthwashes not be used for the prevention of OM in patients undergoing HSCT.
Benzydamine Hydrochloride
As pathogenesis of mucositis primarily involves production of inflammatory cytokines such as TNF-α, anti-inflammatory medications have been studied to reduce the severity of OM.86 Benzydamine hydrochloride (HCl) is an indirect cytoprotectant with anti-inflammatory, analgesic and antimicrobial activity. Benzydamine HCl was studied in a multicenter, randomized, double-blind, placebo-controlled clinical trial for prophylaxis of radiation-induced OM. Benzydamine HCl was used as 15 mL oral rinse for 2 minutes, 4–8 times daily before and during RT, and for 2 weeks after completion of RT. The benzydamine HCl oral rinse significantly reduced erythema and ulceration by approximately 30% compared with the placebo and also delayed the use of systemic analgesics compared with placebo. However, it was found to be ineffective in subjects receiving accelerated RT doses (≥220 cGy/day). Based on this trial and other pervious trials,87,88 the Cochrane review concluded that this agent may have some benefit in preventing or reducing the severity of OM associated with cancer treatment.
- The MASCC/ISOO panel recommends the use of benzydamine for prevention of radiation-induced OM in patients with head and neck cancer receiving moderate-dose radiation therapy (regimens with cumulative doses up to 5000 cGy).
Of note, a recent study sponsored by McNeil was conducted to study the efficacy of benzydamine and was discontinued after an interim analysis and the recommendations of the Data Monitoring Committee.
Amifostine
Amifostine is a thiol drug which is cytoprotective by several mechanisms, including scavenging oxygen-derived free radicals, DNA protection and repair acceleration, and induction of cellular hypoxia. While amifostine has FDA approval to reduce the incidence of moderate to severe xerostomia in patients undergoing postoperative radiation treatment for head and neck cancer,89–91 studies on management of OM have shown conflicting results.92,93 The Cochrane review concluded that amifostine appears to have small benefit in preventing and reducing the severity of mild OM.
- The MASCC/ISOO panel recommends the use of amifostine for the prevention of esophagitis in patients receiving chemoradiotherapy for non–small-cell lung cancer and suggests that intravenous amifostine at a dose of 340 mg/m2 daily prior to radiotherapy may prevent radiation proctitis in patients who are receiving standard-dose radiotherapy for rectal cancer.
However, the panel found that most of the amifostine studies on the reduction of OM have been small, single-center studies with conflicting results.
Glutamine (Saforis® or AES 14)
Glutamine is a nonessential amino acid which is widely distributed throughout the body and becomes an essential amino acid during disease or trauma.94 Glutamine has been shown to improve immunologic function by decreasing the inflammatory response and to improve OM after high-dose chemotherapy followed by autologous HSCT. Multiple trials studying its effect on OM prevention have shown some promise.95–97 In 2007, a Cochrane review concluded that there is insufficient evidence to conclude that glutamine is effective for the prevention of OM formation at any level of severity. The MASCC/ISOO panel concurs and recommends against the use of systemic glutamine for the prevention of gastrointestinal mucositis because of lack of efficacy. Recently, Saforis (MGI Pharma, Minneapolis, MN), a proprietary oral suspension of L-glutamine powder, has shown beneficial effects in OM. It is believed to aid in uptake of glutamine into epithelial cells and may reduce mucosal injury by reducing the production of proinflammatory cytokines and cytokine-related apoptosis.98,99 A Phase III randomized, placebo-controlled trial of Saforis for prevention and treatment of OM in breast cancer patients receiving anthracycline-based chemotherapy showed a significant reduction in incidence of OM.100
Low-Level Laser Therapy
Finally, there is some literature support for the use of low-level laser therapy (LLLT) in reducing the symptoms and severity of OM.101,102 The mechanism of action is not well elucidated, but it is believed that the absorption of laser energy by chromophores on mitochondria or other intracellular organelles results in the upregulation of wound-healing mechanisms. The various studies evaluating LLLT have been difficult to compare owing to different parameters utilized, such as different types of laser sources (HeNe, GaAlAs, and GaAs), wavelengths (632.3, 650, 660, 780, 810–820, and 901 nm), powers (15–70 mW), and energy densities.103
- The MASCC/ISOO panel suggests the use of LLLT to reduce the incidence of OM and its associated pain in patients receiving high-dose chemotherapy or chemoradiotherapy before HSCT.
The panel noted that LLLT requires expensive equipment and specialized training and hence suggests its use only if the treatment center is able to support the necessary technology and training.
12.3 Nonmalignant and Noninfectious Oral Ulcerations
Before discussing the nonmalignant and noninfectious subgroup of oral ulcerative diseases, it must be said that clinical history, careful examination, and laboratory studies including culture and biopsy are critical to making sure you have correctly eliminated the infectious (viral, bacteria, fungal) and neoplastic causes for an oral ulcer. This chapter does not review these methods as there are several excellent articles and textbooks that will help the clinician distinguish between oral mucosal lesions.104–107
12.3.A Description and Classification
Ulcers, by definition, are characterized by a loss of surface tissues and they affect both the epithelium and underlying connective tissue.108 The term “erosion” must be differentiated from ulcers in that erosion involves only superficial epithelium and can be as painful as an ulcer. However, as this chapter focuses on treatment of pain mainly, the term “ulcer” is generally used to include erosions caused by vesiculobullous diseases and other causes. Oral ulcers are very common lesions of the oral mucosa and are generally painful.109 Many authors classify oral ulcers into two main groups: (1) acute ulcers with abrupt onset and short duration, and (2) chronic ulcers (greater than 2 weeks in duration) with slow onset and progression. Unfortunately, the distinction between acute and chronic is not based on etiology and sometimes, when an ulcer is chronic, it may mean that the etiology is simply still present. For example, a patient who has a contact-allergy-induced ulceration will have a chronic presentation if the offending agent is not identified and removed. In this chapter we suggest that the noninfectious and non-neoplastic ulcerative disorders might be better grouped according to their suspected etiologies, not by duration (see Sec. 10.3.C). To assist in differentiating these ulcers, the typical clinical features are presented in tabular form (Table 12.2)
Table 12.2 Nonmalignant and noninfectious ulcers: clinical features
| Oral ulcerative disease | Clinical features |
| Drug-induced ulcers | Single, isolated ulcers, located on the side of the tongue, surrounded by an erythematous halo and resistant to usual treatments |
| Erosive lichen planus | Areas of atrophy, erosions, or painful ulcers, generally resistant to conventional treatments |
| Pemphigus vulgaris | Bullae appear in oral cavity (posterior region), forming painful ulcers with necrotic fundus and erythematous halo. |
| Mucous membrane pemphigoid | Spontaneous onset of bullae that readily rupture, giving rise to a highly painful ulcerated area (most commonly on palate and gingiva) |
| Lupus erythematosus | Erythema and oral ulcers, without induration and accompanied by whitish striae and a tendency to bleeding |
| Reiter’s syndrome | Arthritis, urethritis, conjunctivitis, and oral ulcers similar to those of recurrent aphthous stomatitis |
| Eosinophilic ulcer | Large ulcer, generally in the tongue, with raised, indurated borders and white-yellowish fundus that may resemble a malignant lesion; persists for weeks or months |
| Traumatic ulcer | Ulcers appear in short and painful episodes; white or yellowish central clear area with erythematous halo |
| Recurrent aphthous stomatitis | One or multiple recurrent and painful ulcers; well-defined, round or oval ulcers covered by a white or greyish pseudomembrane and surrounded by an erythematous halo |
| Behçet’s disease | Recurrent oral (aphthae) and genital ulcers, skin lesions, and ocular, musculoskeletal, cardiovascular, gastrointestinal, and neurological symptoms |
| Necrotizing sialometaplasia | Extensive deep ulcers with indurated borders located in hard or soft palate |
| Allergic reactions | Features ranging from erythema to ulceration in oral mucosa |
| Erythema multiforme | Erythema, vesicles, and ulcers in oral mucosa; involvement of the lips in almost all cases, leaving scabs; typical target skin lesions |
| Blood-disease related | Ulcers similar to those of recurrent aphthous stomatitis |
12.3.B Epidemiology
In 2004, using the data from the Third National Health and Nutrition Examination Survey, one study reported on the most common oral lesions in the United States. They found that many of the lesions seen were related to dental prostheses and tobacco use.110 A 2002 study examined the oral mucosa of 500 residents of Thailand who were 60 years of age or older.111 They reported that 83.6% of those examined had some type of abnormality, and traumatic ulcer had an incidence rate of 15.6%. In 2005 a review of the literature described that children have oral lesions with incidence rate ranging from 4.1% to 52.6% of the population, of which the most common were recurrent aphthous stomatitis (0.9–10.8%), labial herpes (0.78–5.2%), and traumatic injury (0.09%–22.15%).112
12.3.C Etiology-Based Subgroups for Noninfectious and Non-Neoplastic Oral Ulcers
We suggest six etiologic groups: (1) trauma, (2) allergic reactions, (3) nonspecific adverse drug reactions, (4) ulcers associated with autoimmune disease, (5) ulcers associated with blood disorders, and (6) idiopathic ulcers. The clinical presentation will vary depending on the allergen, the trauma, or the drug involved. In the following subsections, we present these six subgroups and 15 specific and different oral ulcerative conditions within these groups (see Table 12.3).
Table 12.3 Noninfectious and non-neoplastic oral ulcerative diseases
| Oral ulcerative disease | Etiology |
| Traumatic oral ulcers | External physical trauma, chemicals, electricity, and heat |
| Recurrent aphthous disease | Inflammatory disease of unknown origin |
| Behçet’s disease | Genetic, environmental, infectious, immunological, and hematological factors have been implicated. |
| Necrotizing sialometaplasia | Ischemic injury secondary to trauma or to damage from a chemical or biological agent |
| Allergic contact stomatitis | Contact allergic reaction |
| Erythema multiforme | Late cell-mediated immune reactions to drug |
| Ulcers related to blood diseases | Associated with blood deficiencies (anemias, leukemias, lymphomas, multiple myeloma, and neutropenias) |
| Drug-induced oral ulcers | Allergic reaction to drugs |
| Lichen planus | Autoimmune disease (T-cell-mediated attack on basal keratinocytes) |
| Pemphigus vulgaris | Autoantibodies to desmosomal proteins |
| Mucous membrane pemphigoid | Autoimmune disease |
| Lupus erythematosus | Autoimmune disease of the connective tissue. |
| Reiter’s syndrome | Autoimmune disease |
| Eosinophilic ulcer | Etiology uncertain, but associated with traumas |
Trauma-Induced Oral Ulcers (Subgroup 1)
Direct Trauma
Clinical Presentation
Depending on the nature of the traumatic injury, the location, depth, and appearance of the ulcer will vary. As a general rule, however, traumatic ulcer is characterized by acute pain of moderate intensity and by a white or yellowish central clear area with an erythematous halo.
Causation
The most common causative agents are external physical trauma, chemicals, electricity, and heat. In addition, self-inflicted trauma, caused by sharp teeth and tooth edges or a chewing incoordination, can produce oral tissue damage and ulcers. Ill-fitting dental prothestic devices can cause chronic mucosal reactions due to localized pressure and simple friction to the tissues.113 Some patients have a greater predisposition to oral mucosal trauma than others, namely, older patients and diabetes mellitus patients. Biting of the tongue or lower lip after dental anesthesia can be the source of self-induced mucosal trauma as well as incorrect or too aggressive tooth brushing.114 Some medications can be caustic enough to induce ulcers via direct contact. While we will discuss separately the nonspecific adverse mucosal tissue reactions associated with systemic medications in section covering subgroup 3, there are also caustic chemical reactions of the mucosal tissues due to direct and prolonged contact of various medications on the tissues (e.g., aspirin tablets held in the mouth).115–117 Oral ulcerations can also occur from illegal drugs that come into prolonged contact with oral tissues (e.g., cocaine).
Necrotizing Sialometaplasia
Clinical Presentation
Necrotizing sialometaplasia is an uncommon lesion which presents as a large deep ulcer with indurated borders that is located on the hard or soft palate, without obvious traumatic injury. It is commonly mistaken as a malignant neoplasm but actually is a self-limiting and benign necrotizing inflammatory disease of the minor salivary glands.118
Causation
The cause is believed to be an ischemia secondary to trauma or to damage from a chemical or biological agent. Local anesthesia, thermal trauma due to smoking, traumatic tissue injury, surgical trauma, upper respiratory infection, and allergies have been pointed out as etiological agents. These injuries are theorized to affect the vascular system, causing an ischemia in the salivary glands that may result in local tissue necrosis.119
Ulcers Induced by Allergic Reactions (Subgroup 2)
Allergic Contact Stomatitis
Clinical Presentation
Allergic contact stomatitis usually presents with edema, erythema, ulcer, hyperkeratotic changes, or a burning sensation. These reactions can range from mild erythematous changes in the oral mucosa (with or without ulceration) to severe ulcerative blistering reactions. In most cases, the diagnosis of this problem is not actually based on the clinical characteristics of the tissue changes but on a careful detailed medical history that includes gathering information about all oral products, foods, or medications being used.
Causation
Contact stomatitis is very commonly due to cinnamon-flavored chewing gum or other cinnamon-flavored dental products. There are numerous other food substances and medicines that come into contact with the oral mucosa and, in susceptible patients, can induce a contact allergic reaction in the mouth.120 Restorative materials that contain mercury are known to be able to induce an allergic reaction called a lichenoid lesion in the contacting tissue. While a local lichenoid lesion can be distinguished by its location it may not be histologically distinguished from oral lichen planus.121–124 The most common location of oral lichenoid lesions are the buccal mucosa and lateral tongue borders adjacent to the suspected causative restoration. With oral lichen planus, the tissue changes are not limited to those sites that are in direct contact with restorations and many patients may have cutaneous or other mucosal sites with lesions (e.g., skin or vulvo-vaginal mucosa).125 When a direct contact allergy is suspected it is possible to perform cutaneous patch testing using various dental restorative materials in the test. This test is normally on the skin of the arm or back but the validity of this testing process is questionable.126–130 At present data suggests that mercury-containing restorations are more prone to allergic reactions, but some reports also exist regarding allergic reaction to gold, porcelain, composite, and glass ionomer cements as well.131
Erythema Multiforme and Its Subtypes
Clinical Presentation
This allergy-induced vesiculobullous mucocutaneous disease is potentially life-threatening and is diagnosed only after exclusion of other diseases. It can present in one of three forms.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


