Chapter 12
Minimal and Moderate Sedation Agents
Sedation usually implies a modification of the level of consciousness of an individual, ideally resulting in a state of lessened anxiety or fear, relaxation, and sometimes favorable mood enhancement. The change in consciousness can be induced through non-pharmacological or pharmacological intervention. This chapter will focus solely on pharmacologically-mediated changes in consciousness.
Sedative medications alter the level of consciousness of an individual. The level of consciousness is represented as a continuum ranging from full wakefulness to complete coma and is dependent, to a degree, on the number and dose of pharmacological agents administered to the individual. Hence, the level or depth of sedation is often referred to as an indirect, continuous index of the patient’s level of consciousness at any given point in time.
There are many ways to define levels or depths of sedation. Nonetheless, definitions of sedation are found in sedation guidelines offered by various professional organizations (American Dental Association 2007; American Academy of Pediatric Dentistry 2006; American Society of Anesthesiologists 2002). The most frequently used guidelines for sedation of the pediatric patient in any setting including dentistry is that of the current American Academy of Pediatrics/American Academy of Pediatric Dentistry (AAP/AAPD). Three different levels of sedation are defined in those guidelines:
Minimal (old terminology of “anxiolysis”): a drug-induced state during which patients respond normally to verbal commands. Although cognitive function and coordination may be impaired, ventilatory and cardiovascular functions are unaffected.
Moderate (old terminology “conscious sedation” or “sedation/analgesia”): a drug-induced depression of consciousness during which patients respond purposefully to verbal commands (e.g., “open your eyes,” either alone or accompanied by light tactile stimulation—a light tap on the shoulder or face, not a sternal rub). For older patients, this level of sedation implies an interactive state; for younger patients, age-appropriate behaviors (e.g., crying) occur and are expected. Reflex withdrawal, although a normal response to a painful stimulus, is not considered to be the only age-appropriate purposeful response—it must be accompanied by another response, such as pushing away the painful stimulus so as to confirm a higher cognitive function. With moderate sedation, no intervention is required to maintain a patent airway, and spontaneous ventilation is adequate. Cardiovascular function is usually maintained. However, in the case of procedures that may themselves cause airway obstruction (e.g., dental or endoscopic), the practitioner must recognize an obstruction and assist the patient in opening the airway. If the patient is not making spontaneous efforts to open his airway and relieve the obstruction, then he should be considered to be deeply sedated.
Deep: a drug-induced depression of consciousness during which patients cannot be easily aroused, but respond purposefully (see discussion of reflex withdrawal above) after repeated verbal or painful stimulation (e.g., purposefully pushing away the noxious stimuli). The ability to independently maintain ventilator function may be impaired. Patients may require assistance in maintaining a patent airway, and spontaneous ventilation may be inadequate. Cardiovascular function is usually maintained. A state of deep sedation may be accompanied by partial or complete loss of protective airway reflexes.
In this chapter, the focus is on minimal and moderate sedation. However, it is important to remember that any sedative drug and its dose can produce variable levels of sedation. It is therefore impossible and inappropriate to refer to any one drug as a “drug that produces minimal sedation.” Indeed, it may produce a state of minimal sedation in most children, but others may respond either in a less (hypo-responder) or a more (hyper-responder) exaggerated fashion than expected.
A major theme and important concept in the AAP/AAPD sedation guidelines is that of rescue. Rescue, as used in the guidelines, refers to a practitioner’s knowledge, training, and skills in providing competent management for the patient who is in the process of or could potentially drift into a compromised condition. Ultimately, any practitioner who sedates a child for a procedure must be able to recognize any compromised state of the child and act immediately to stabilize the patient and prevent a disastrous outcome. The most frequently occurring compromised state resulting from sedation in children is respiratory depression. Therefore, practitioners must be efficient and effective in basic airway management skills, including the use of positive pressure oxygen with a bag-valve-mask. It is, therefore, essential that specialty training programs provide appropriate experiences for trainees before they are allowed to sedate children. Training may best be obtained by dedicated anesthesiology rotations in which trainees are frequently and directly exposed to compromised respiratory conditions and mentored by highly trained, skilled professionals.
Drugs
Drugs have been used to sedate children for dental procedures for well over a century. Isolated reports reviewing drugs as “premedications” for children during dental procedures can be found in the literature of the 1950s and 1960s. The same drugs, other than alcohol, paralleling that era were also used in medicine for various conditions with barbiturates dominating for almost the first half of the 20th century (Lopez-Munoz et al. 2005). Other notable drugs of that time were chloral hydrate, opiums (primarily morphine), and bromides.
In 1952, Ruble conducted a classic review of that era’s agents. He described the current literature of the primary and popular drugs, including barbiturates, bromides, and morphine. Interestingly, as described in his review, the issues and challenges of the 1950s remain consistent with those of today. The premedication was indicated for the “nervous and highly apprehensive child.” Concern revolved around the depth of sedation, dose, and route of administration. Family guidance at home, school, and in the dental office, or the lack thereof, resulted in the “happy” versus “maladjusted” individual. It was noted that a “screaming, violent child” made relatively simple procedures difficult and time-consuming for the dental team, and that sedation was “helpful to the child and the dentist.” Other studies and written opinions of the day addressed these issues as well (Aduss et al. 1961; Album 1955; Buckman 1956; Lampshire 1950).
There have been eleven surveys over the past four decades identifying several agents used to sedate children for dental procedures. They are listed with the references at the end of the chapter. The more common agents identified were nitrous oxide, chloral hydrate, meperidine, midazolam (and other benzodiazepines), and hydroxyzine (and other antihistamines). Agents such as morphine, alphaprodine, barbiturates, and chlorpromazine have been mentioned as well (Brandt and Bugg 1984; Doring 1985; Lambert et al. 1988; Myers and Shoaf 1977; Riekman and Ross 1981; Roberts et al. 1992). The more common agents fall into four categories: hypnotics, benzodiazepines, antihistamines, and inhalation agents. This chapter will focus on the oral route for these agents.
Hypnotics
Hypnotics are drugs that promote drowsiness and sleep, and are generally classified as barbiturates and non-barbiturate types. Barbiturates such as pentobarbital were popular decades ago. However, because of their potential to create paradoxical reactions, they are no longer favored as sedating agents for children.
Chloral Hydrate
For decades, the most common hypnotic agent used in pediatric dentistry has been chloral hydrate. Although chloral hydrate can be and has been used as a single agent (Anderson 1960; Czaarnecki and Binns 1963), most recent studies have investigated chloral hydrate in combination with one or more additional agents. More than twenty-five of these studies have been included in the reference list at the end of this chapter.
Characteristics
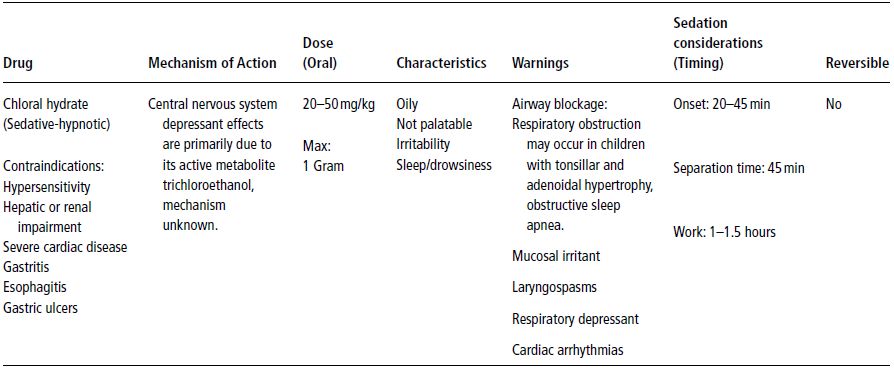
Chloral hydrate was discovered in 1832 by Justus Liebig and was introduced in medicine as an anesthetic and hypnotic drug in 1869 (Stetson 1962). It acts by depressing the central nervous system. Its mechanism of action is not well understood, but it is thought to involve the GABA receptor complex (Lu and Greco 2006). As a hypnotic, therapeutic doses of chloral hydrate can cause sleepiness, drowsiness, or, in some cases, hyperactivity. Care must be exercised whenever using chloral hydrate in combination with other agents, as the depth of sedation may increase and respiratory depression can occur. A unique effect of chloral hydrate is the potential inhibition of the tongue’s genioglossal muscle (Hershenson et al. 1984). Children with large tonsils (see Chapter Ten) and adenoid tissue may not be appropriate chloral hydrate recipients because of the increased likelihood of upper airway blockage, especially when the patient is prone.
Chloral hydrate is an oily substance and a noted irritant to mucosal tissue. Hence, it should not be used in patients who have conditions involving gastritis, esophagitis, or oral lesions. Care must also be taken to avoid contact of chloral hydrate with the conjunctiva of eyes, which may occur when orally administering chloral hydrate when the patient coughs or spits. Rapid administration of chloral hydrate in children using a needleless syringe with splashing against the posterior portion of the mouth should also be avoided. Chloral hydrate in higher doses has also been associated with cardiac dysrhythmias; therefore, its use should be avoided in patients with certain cardiac conditions.
Chloral hydrate has no analgesic properties. It has an unpleasant taste and usually requires a flavoring vehicle when orally administered. The formulation and production of the oral solution of chloral hydrate in the United States was ceased in April 2012, but it remains available in other countries. Other formulations of chloral hydrate (e.g., capsules) are still available in the United States, however, and oral solutions of chloral hydrate can be formulated by local pharmacists, should they elect to do so.
Case 12.1, Discussion: One of the very challenging aspects of using minimal-sedation oral sedatives is the administration of the drug. The majority of patients undergoing minimal sedation are uncooperative, and at many times exhibit defiant behavior. Failure to ingest the prescribed dose of medication will inevitably result in a less-than-optimal sedation session. In many instances, parents have difficulty administering the syrup. When the dentist is faced with a situation similar to the one described above—when he is unsure of how much of the drug has been consumed—it may be hazardous to administer more of the drug in order to continue with the appointment.
The dentist should first offer the parent the option of giving their child the drug, explaining why the child must swallow the entire dose. The child may be coaxed by the parent into taking the relatively small amount of syrup, followed by a minute amount of water. A cup or syringe should be offered. Some children will be more willing to drink the syrup from a cup rather than a syringe. However, in many instances the parent will fail. Even if this result is anticipated, it may be wise to allow the parent to fail, as this will facilitate consent to the drug’s administration by the dentist.
Figure 12-1. The dentist embraces the child’s head (a) and slowly dribbles the solution down and off thefinger or thumb of the non-dominant hand that is strategically placed on the retromolar pad of the patient (b). This usually stimulates the swallowing reflex and gives the child a chance to coordinate breathing and swallowing.
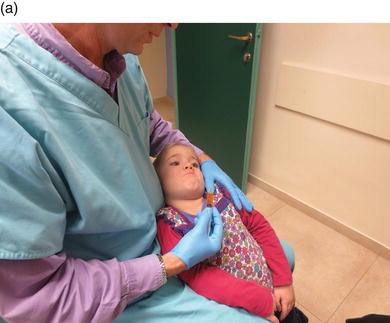
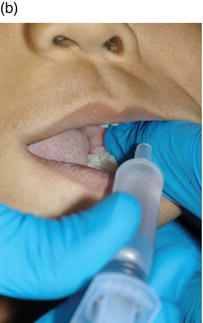
Once consent to administer the drug is given, stand the child in front of the sitting dentist. The child’s head is tilted backward. The parent restrains the child’s hands. The dentist embraces the child’s head and slowly dribbles the solution down and off the finger or thumb of the non-dominant hand that is strategically placed on the retromolar pad of the patient (Figure 12-1). This usually stimulates the swallowing reflex and gives the child a chance to coordinate breathing and swallowing. Sometimes the child refuses to swallow and a pool of solution begins to form in the oro-pharynx; at that time, the parent is instructed to pinch off the nose briefly to either cause swallowing or expectoration (usually the former). Too often children have a very difficult time coughing and managing their airway reflexes when too much solution is shot into the mouth (usually by a parent) or too rapidly administered by the doctor.
This technique is especially important when administering chloral hydrate, due to is mucosal irritation. It is always possible to induce a partial laryngospasm if chloral hydrate is shot off the back pharyngeal wall and bounces down around the epiglottis and laryngeal structures.
Clinical Perspective
Typically, children exhibit slight disinhibition or excitement within the first 15–25 minutes following oral administration of chloral hydrate or a combination of agents dominated by chloral hydrate. Sometimes the disinhibition is exhibited as talkativeness, exploratory hyperactivity in the environment, social interaction, and general silliness, but it can show itself as occasionally frank agitation. This phase is usually followed by drowsiness or sleepiness and can result in sleep itself. The latter phase is not sufficiently established to the point that one can begin patient separation from the parent to start dental procedures, but does require careful monitoring clinically and with electronic monitors (e.g., pulse oximetry), depending on the growing depth of sedation.
Parents are generally dismissed before the procedure starts. Separation from the parent should not begin until approximately 45 minutes after its administration, at which time sufficient blood levels of the active metabolite begin to prevail. The working time (depending on whether other drugs are “on-board”, the patient’s level of natural fatigue, and the child characteristics such as temperament and cognitive development) is usually 60 minutes or more.
It should be noted that clinical technique and protocol are very important throughout the entire treatment. Many clinicians use oral premedication sedation together with nitrous oxide sedation; details of its administration and use have been described in the preceding chapter. Following the placement of the nasal mask, begin with slow, deliberate movements to open the airway. Point the chin of a supine patient toward the ceiling. The clinician can distract the child with chatter using a low voice if the child is awake. Following proper titration of nitrous oxide concentration and flow, gently open the mouth slightly, insert a mouth prop, and slowly open the mouth wider. After reviewing and confirming the planned treatment, topical and local anesthesia are administered. Chapter Eight describes in detail the administration of local anesthesia. If the child becomes agitated during the injection, the clinician should “re-settle” the child once local anesthetic is administered.
A rubber dam or a comparable method (e.g., Isolite, but not cotton roll isolation or no isolation) should always be used for sedations. Generally, one can cut teeth dry or use a very light water spray that is rapidly suctioned from the mouth with high-speed suction. (Note: the high-speed suction should initially be activated at some distance from the patient and slowly brought closer so as to not startle the patient.) The same is true for the overhead lamp. Activate it away from the patient’s face and slowly adjust it to illuminate the mouth. Tooth preparation can begin once adequate anesthesia is obtained. When working efficiently the restorative phase can be completed quickly, although occasionally a child may become agitated and need to be re-settled. If this sequence of events is followed, and the patient begins the procedure in a non-agitated state, it usually results in a good sedation outcome. This procedure can also be followed with other sedatives.
One of the earlier chloral hydrate studies was that of Anderson in 1960. He used chloral hydrate alone when providing dental care to children. Anderson advocated the use of chloral hydrate to “make a difficult, emotional patient easy to work on” and to help with patient tolerance. He indicated that up to 5 teaspoons (1200 mgs) was necessary for some three- to four-year-old patients 30 minutes before dental treatment. He reported on 300 patients’ sedations, indicating that often local anesthesia was unnecessary and all dental treatment could be accomplished in one appointment. Other studies using chloral hydrate as the single agent or with nitrous oxide have also been reported (Barr et al. 1977; Houpt et al. 1985; Moore et al. 1984). Most of the studies indicate that chloral hydrate produces good to excellent sedations. However, chloral hydrate currently is rarely used as a single agent for children during dental procedures.
No fewer than twenty-five studies have documented the use of choral hydrate in combination with other sedatives, particularly antihistamines. These studies have been included in the references at the end of the chapter. The dosage range used in these studies for chloral hydrate and hydroxyzine is 40–75 mg/kg and 1.0 mgs to 2 mg/kg, respectively. There is some support to the expectation that the addition of hydroxyzine to chloral hydrate improves patient behavior, compared to chloral hydrate alone (Avalos-Arenas et al. 1998), but others have found no improvement (Needleman et al. 1995).
Promethazine has also been a popular agent as a sedative with antihistaminic properties that has been used with chloral hydrate (Dallman et al. 2001; Houpt et al. 1985; Lu and Lu 2006; Robbins 1967; Sams et al. 1993; Sams and Russell 1993; Wright and McAulay 1973). Its dose has been reported in these studies by body weight (1 mg/kg) and as a single bolus (12.5 mg). Blood pressure may be slightly lower in this combination compared to midazolam or chloral hydrate and meperidine (Dallman et al. 2001; Sams and Russell 1993), but the effect is not perceived as clinically significant.
Table 12-1. Chloral Hydrate.
Chloral hydrate has also been used in combination with meperidine and hydroxyzine. This combination has been anecdotally known as a “triple” combination. It is still taught in advanced pediatric dentistry training programs and has remained fairly popular (Wilson and Nathan 2011). Generally, when compared to other sedatives or drug combinations, this combination tends to cause improved behavior, interpreted as increased quiet and decreased crying behaviors (Chowdhury and Vargas 2005; Hasty et al. 1991; Nathan and West 1987; Wilson et al. 2000). However, this is not always the case, as dose differences or other similar “triple” combinations have shown no improvement in behavior or equivalent outcomes (Poorman et al. 1990; Sheroan et al. 2006). It is possible that the dose of chloral hydrate used may make a significant difference in the behavioral outcomes, with a higher dose mediating a greater likelihood of quiet/sleep behaviors. Nonetheless, with a greater likelihood of quiet/sleep behaviors comes a higher risk of airway or respiratory compromise.
There may be an increased risk of respiratory compromise manifested as apnea and/or oxygen desaturation when the triple combination involves chloral hydrate at a dose of 50 mg/kg (Croswell et al. 1995; Leelataweedwud and Vann 2001; Leelataweewud et al. 2000; Rohlfing et al. 1998; Sheroan et al. 2006). It is possible that less respiratory compromise may result by lowering the dose of chloral hydrate and increasing that of meperidine or substituting midazolam for chloral hydrate in the triple combination (Chowdhury and Vargas 2005; Sheroan et al. 2006). A summary of the characteristics, mechanism of action and dosage of chloral hydrate is presented in Table 12-1.
Meperidine
Meperidine has been the most commonly used narcotic in pediatric dentistry, although it is rarely used alone (Cathers et al. 2005; McKee et al. 1990; Song and Webb 2003). At least eighteen studies, have documented meperidine combined with other sedative agents such as midazolam, hydroxyzine or promethazine, and chloral hydrate with hydroxyzine. These studies are included with the references at the end of this chapter. One of the primary reasons to use meperidine in combination with another sedative agent is its analgesic properties, as most other agents with which it is combined, such as midazolam, usually lack such properties. Additionally, meperidine can slightly potentiate the sedative effect of another agent (Chowdhury and Vargas 2005; Nathan and Vargas 2002; Wilson et al. 2000), and in many cases gives the impression of altering the mood of the patient.
Table 12-2. Meperidine (Demerol, Pethidine).
A major drawback to this agent is its likelihood to cause respiratory depression and hypotension. This is particularly true when administered parenterally, with a lessened risk anticipated when delivered via the oral route. Its use in combination with other sedatives should be carefully assessed because of the additive or synergistic properties of sedative agents.
Narcotics, including Demerol, should be used with caution with local anesthetics. The threshold level for seizures is apparently lowered when both are used in combination.
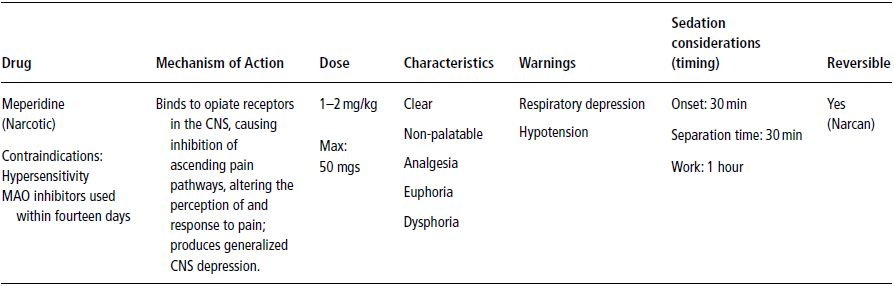
Meperidine is often administered orally, but due to its bitter taste, it requires some masking with a flavoring agent. The submucosal route is another popular means of administering meperidine (Cathers et al. 2005; Chen et al. 2006; Lochary et al. 1993; Roberts et al. 1992; Song and Webb 2003). One study evaluated the behavior of children receiving dental care under sedation with meperidine administered orally versus submucosally. There were no differences in behavior based on the route of administration (Song and Webb 2003).
Generally, the onset of meperidine effects is quicker when administered submucosally, compared to oral administrations. One drawback of submucosal administration is that it can elicit a hyperemic effect often resulting in a “wheal” and itchiness over the facial area where the injection was given. These effects are indirectly triggered by histamine release from mast cells in addition to the vascular effects directly caused by exposure to meperidine (Flacke et al. 1985; Flacke et al. 1987; Levy et al. 1989). Another possible side effect of administering meperidine submucosally is that injection into a large venous complex, just distal to the maxillary tuberosity, can potentially cause rapid onset of hypotension. Considering these cautions, it seems more prudent to administer meperidine in therapeutic doses via the oral route, which tends to eliminate the submucosal effects. Another serious concern is potential interaction between local anesthetics and some narcotics, including meperidine. Excessive use of either or both can result in seizures and/or death (Moore and Goodson 1985). A summary of the characteristics, mechanism of action and dosage of meperidine is presented in Table 12-2.
Benzodiazepines
Benzodiazepines are a large class of drugs that tend to have a fairly wide margin of safety when used alone and in therapeutic doses. They have several properties which are beneficial to many conditions and generally cause, to relative degrees, anti-anxiety, sedative-hypnotic and anticonvulsant activity, skeletal muscle relaxation, and amnestic effects. Their mechanism of action is associated with activation of the GABA receptor complex, which, when activated, has a generalized inhibitory effect. Thus, benzodiazepines indirectly tend to increase the inhibitory action of GABA. Although there are many benzodiazepines on the market, the most frequently reported benzodiazepines used for sedating children for dental procedures are midazolam, diazepam, and triazolam.
Midazolam
Midazolam is purportedly the most popular sedative agent and benzodiazepine for children undergoing dental and medical procedures (Bhatnagaret et al. 2012; Isik et al. 2008; Wilson and Nathan 2011). It was first used as a sedative for dental treatment in the early 1990s (Roelofse and de Joubert 1990) and has been used in medicine since the early 1980s (Haas et al. 1996). When its popularity rose in dentistry, midazolam was reviewed, noting its development, characteristics, metabolism, use in studies, and adverse events (Kupietzky and Houpt 1993).
Clinical Perspective
The sequence of behavioral events that occurs after the oral administration of midazolam is as follows. Slight but perceptible changes in attitude and even activity can be seen within 5 minutes. In 10–15 minutes, significant relaxation occurs and increased socialization is noticeable. Sometimes the child is overcome by a more quiet but friendly mood, especially if they were initially shy or withdrawn. Somewhere between 15–20 minutes after the child has received midazolam, separation of the child from the parent can take place. If nitrous oxide is to be used as a co-medication, begin to place the nitrous oxide hood over the patient’s nose, again using conversation as a distraction.
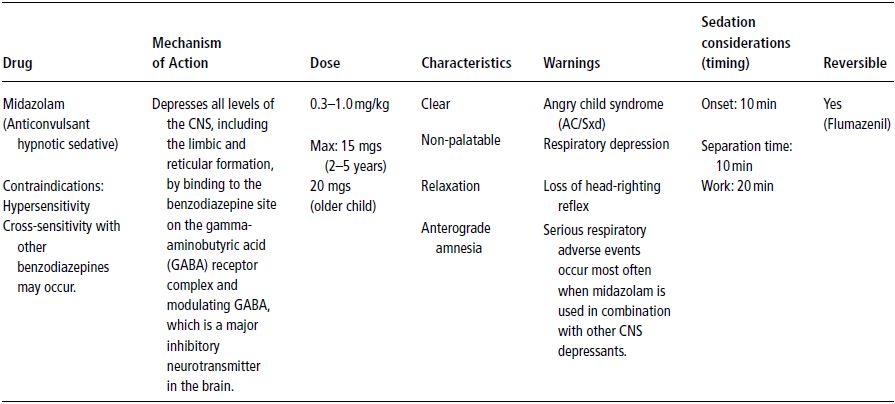
As mentioned previously with chloral hydrate, the same protocol is used for settling the child and starting restorative care. Unfortunately, the working time for midazolam is only 20–40 minutes. So midazolam, when used alone, can be used only for short dental procedures. Occasionally, frank agitation and paradoxical excitement will occur in a small fraction of patients, resulting in an inconsolable, unmanageable child, even in the arms of the parent. This response usually occurs immediately following or during a painful procedure. Anecdotally, this type of response has been referred to as the “angry child syndrome.”
Midazolam has been used alone during dental procedures with approximately two-thirds of the patients reportedly accepting dental treatment successfully (Erlandsson et al. 2001). Others have demonstrated midazolam’s improvement in patient attitude, behavior, and general procedural outcome compared to a placebo or pre-sedation behavior (Gallardo et al. 1994; Mazaheri et al. 2008; Wan et al. 2006).
Midazolam has been used in combination with meperidine, hydroxyzine, ketamine, chloral hydrate, tramadol, fentanyl, sufentanil, nalbuphine, droperidol, and acetaminophen (Cagiran et al. 2010; Heard et al. 2010; Milnes et al. 2000; Myers et al. 2004; Nathan and Vargas 2002; Padmanabhan et al. 2009; Reeves et al. 1996). Few of these studies are alike in protocol or study design; thus, it is almost impossible to determine what combination is consistently superior, if any. Nonetheless, when another agent is added to midazolam, the combination usually results in a slight improvement of behaviors compared to midazolam alone (Al-Zahrani et al. 2009; Cagiran et al. 2010; Nathan and Vargas 2002; Shapira et al. 2004), but not always. The improved behavior may be a function of doses (Musial et al. 2003).
Midazolam is typically administered orally to small children for dental procedures. However, the intranasal route of administration has also received attention from researchers. The most recent investigations reported are those of Bahetwar et al. (2011), Heard et al. (2010), Johnson et al. (2010) and Wood et al. (2010). Earlier studies have been included in the list of references. Other routes of administration include intramuscular (Capp et al. 2010; Lam et al. 2005), submucosal (Myers et al. 2004), and intravenous (Arya and Damle 2002). The dose range for midazolam given parenterally (i.e., via any route other than oral and rectal) is much less compared to that of the oral route (e.g., 0.2–0.3 mg/kg versus 0.5–1.0 mg/kg, respectively). As with other agents, including midazolam, a child’s temperament has been shown to be associated with pharmacological outcomes. Shy or withdrawn children tend to have less favorable outcomes (Arnrup et al. 2003; Isik et al. 2010; Jensen and Stjernqvist 2002; Lochary et al. 1993; Primosch and Guelmann 2005). Usually, the first dramatic physiological change, manifested as a higher heart rate and disruptive behaviors, is followed by a quiet, favorable mood. Midazolam lacks analgesic properties; hence, when analgesics are used in combination with midazolam, the behavioral outcomes generally improve (Nathan and Vargas 2002). A summary of the characteristics, mechanism of action and dosage of midazolam is presented in Table 12-3.
Other Benzodiazepines
Diazepam is a commonly used agent in pediatric dentistry, and it is likely that triazolam is used more frequently than is reported. Diazepam produces good skeletal muscle relaxation and anti-anxiety effects. It has a long onset time, usually approaching one hour after its administration before a patient is ready for dental procedures. It also has a good hour of working time, and an even longer period is required before it is fully metabolized and eliminated from the body. Therefore, time to discharge may be prolonged with diazepam, and it may not be very useful in small children in a busy office setting.
There are at least eleven reports of diazepam used as a single agent, as well as with other sedatives. For the reader’s convenience these reports are listed in the references. Several studies have evaluated the effects of diazepam administered rectally in children for dental procedures (Flaitz et al. 1985; Jensen and Schroder 1998; Jensen et al. 1999; Lowey and Halfpenny 1993; de Roelofse and van der Bijl 1993). Most of the studies are older, suggesting that rectal administration is not as popular as it has been in the past. Additionally, some of the studies indicated that midazolam was better than diazepam when administered rectally. One interesting study evaluated the amnestic effect of diazepam administered orally (Jensen and Schroder 1998). Apparently, the amount of amnesia was significantly reduced in the subset of patients who exhibited behavior management problems. Others have had similar results (Sullivan et al. 2001). Further study needs to elucidate whether or not there is an association between disruptive behaviors in young children and amnesia with diazepam and other benzodiazepines.
Diazepam also has been used in combination with ketamine (Okamoto et al. 1992; Reinemer et al. 1996; Sullivan et al. 2001). In these studies, the dose of ketamine was varied between 4–10 mg/kg, given orally. The lower dose was the least successful, and the higher doses were not significantly different from one another; however, a high rate of vomiting was frequently associated with ketamine (Reinemer et al. 1996; Sullivan et al. 2001). A summary of the characteristics, mechanism of action and dosage of diazepam is presented in Table 12-4.
Table 12-3. Midazolam (Versed, Dormicum).
The major risks associated with high doses are hypoventilation and associated hypoxemia. There are interactive effects when used in patients who are on other types of drugs, such as erythromycin (producing unconsciousness), and thus should be used very cautiously under such circumstances.
In therapeutic doses, its effect on the cardiovascular system is negligible; however, higher doses produce decreased blood pressure and cardiac output.
Occasionally in children, the expected sedation does not occur, but rather, a paradoxical hyperactivity occurs and is called the “angry child syndrome.“
Several studies involved children for dental procedures using triazolam. These were done early in the late 1990s and early 2000s. One study evaluated triazolam versus chloral hydrate and hydroxyzine, primarily in preschoolers. The doses were 0.2 mg/kg for triazolam and 40 mg/kg and 25 mgs for chloral hydrate and hydroxyzine, respectively. There were no significant differences in behavior or physiology between the two regimens and the authors suggested that triazolam was just as effective as the more traditional regimen of chloral hydrate and hydroxyzine (Meyer et al. 1990). Interestingly, a report comparing triazolam (0.3 mg/kg) to a placebo in a well-controlled study showed little improvement with triazolam over the placebo (Raadal et al. 1999). Also noteworthy is that triazolam can potentially produce ataxia and visual disturbances in young children as the dose increases from 0.005 to 0.03 mg/kg (Coldwell et al. 1999). Similar findings were reported in slightly older children with triazolam when administered sublingually (Tweedy et al. 2001).
Other non-benzodiazepine like sedative agents have been used for dental procedures in children, including zolpidem (Ambien®), a sleeping aid for adults (Bhatnagar et al. 2012; Koirala et al. 2006). Zolpidem activates a portion of the GABA complex to aid in initiating sleep and can be reversed by Flumazenil. At least two articles have indicated that zolpidem is not a preferred agent in children when compared to other, more commonly used agents (e.g., midazolam).
Antihistamines
Antihistamines are one of the most frequently used adjuncts, second to nitrous oxide, when combined with other sedative agents during sedations for pediatric patients undergoing dental procedures. They also are very popular for mild sedation when used alone and tend to be relatively safe for children (Faytrouny et al. 2007; Shapira et al. 1992). Antihistamines are noted to have antiemetic, drying, and mild sedative properties.
Many studies indicate that the addition of hydroxyzine to another sedative may or may not always improve behavior (Avalos-Arenas et al. 1998; Cathers et al. 2005; da Costa, et al. 2007; Lima et al. 2003; Shapira et al. 2004). This inconsistency in showing a beneficial effect associated with the mix of hydroxyzine with other agents may be due to differences in methodology (e.g., dose). Nonetheless, it remains a popular drug combination for sedating children, most likely because of its antiemetic properties and slight sedative effects, whether it is truly beneficial or not.
Table 12-4. Diazepam (Valium).
In therapeutic doses, the effect on the cardiovascular system is negligible; however, higher doses produce decreases in blood pressure and cardiac output.
Respiratory depression occurs with increased dosages (or repeated doses) or when diazepam is used in combination with other sedative agents (e.g., opioids); otherwise, there is little respiratory effect. Occasionally in children, the expected sedation does not occur, but rather, a paradoxical hyperactivity occurs. This may be accompanied with rage, hostility, and nightmares.
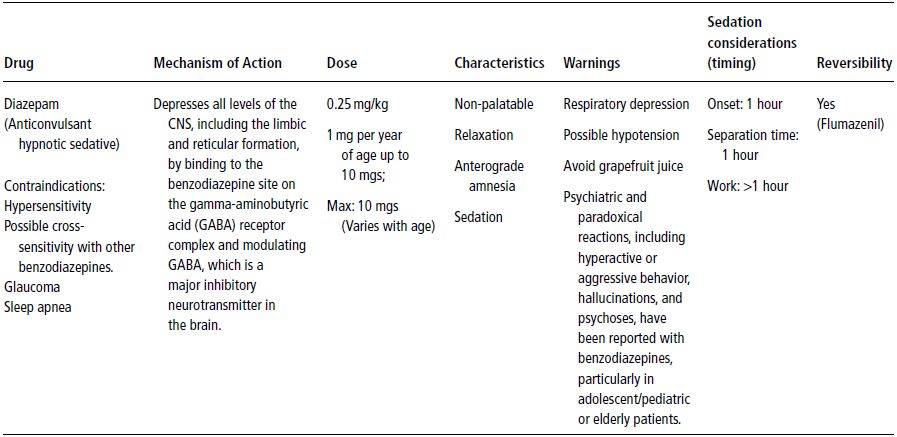
Table 12-5. Hydroxyzine (Atarax or Vistaril).
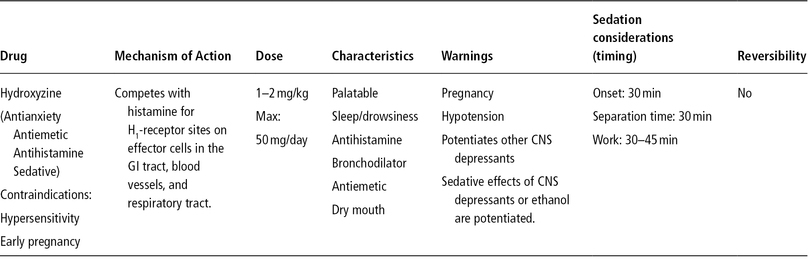
Promethazine has also been a very popular agent used in combination with other agents (Bui et al. 2002; Campbell et al. 1998; Houpt et al. 1985; Myers and Shoaf 1977; Sams et al. 1993; Singh et al. 2002; Song and Webb 2003), but it has not been shown definitively to be more or less effective than hydroxyzine. Furthermore, promethazine has been associated with respiratory depression in children less than two years of age, resulting in an FDA-issued a warning against its use in very young children. Summaries of the characteristics, mechanisms of action, and dosages of hydroxyzine and promethazine are presented in Tables 12-5 and 12-6, respectively.
Diphenhydramine administered orally has not been studied as a sedative agent for children/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


