Chapter 11
Sinus Augmentation Using Tissue-Engineered Bone
Implant placement in the edentulous maxilla often represents a clinical challenge due to insufficient bone height after crestal bone resorption and maxillary sinus pneumatization. Surgical approaches that were developed over the past years aim to restore bone height in the posterior maxilla to create a sufficient implant bed. Boyne and James (1980) were the first to describe a procedure that uses existing space in the maxillary sinus by lifting up the Schneiderian membrane from its bony surface and filling this newly created space with augmentation material. Several modifications of the originally described surgical procedure were developed; however, the basic principle of increasing maxillary bone height by placing graft material in the maxillary sinus after detaching the Schneiderian membrane remained the same (Davarpanah et al. 2001, Fugazzotto and De Paoli 2002, Summers 1994). Grafting materials used to augment bone height in the posterior maxilla can be categorized into four groups: autogenous bone, allografts (from human), xenografts (from a nonhuman species), and alloplasts (synthetic materials). Autogenous bone is the only grafting material with an osteogenic potential, and it has been shown that its use in sinus augmentation can achieve predictable results. Furthermore, autogenous bone requires shorter healing times (4 months versus 8 to 10 months) because it contains living cells and growth factors. Unfortunately, its availability is limited due to anatomical confines and donor site morbidity (Block and Kent 1997, Cammack et al. 2005, Froum et al. 1998, Garg 1999, Pikos 1996, Wheeler 1997). Several current approaches aim to overcome those boundaries; one novel approach uses concepts previously established in the field of tissue engineering. In contrast to conventional one-dimensional in vitro cell culture, tissue-engineering techniques aim to mimic an in vivo environment by using scaffolds, which arrange cells in a three-dimensional fashion (Risbud 2001). Living tissues that otherwise would be limited in their potential to grow can be contained and even expanded in vitro before being reintroduced in vivo.
Periosteum, a membrane that closely enfolds bone, consists of connective tissue and contains chondroprogenitor and osteoprogenitor cell populations (Hutmacher and Sittinger 2003). It has been shown that these progenitor cells can be isolated and stimulated in vitro to form cartilage and bone using tissue-engineering techniques (Arnold et al. 2002, Breitbart et al. 1998). In 2003, Schmelzeisen and colleagues described a clinical procedure that substitutes autogenous bone graft material with tissue-engineered bone in a sinus augmentation procedure. Periosteal tissue was harvested from the oral cavity, and its progenitor cells were isolated and expanded in a three-dimensional bioabsorbable polymer fleece matrix in vitro. The matured transplants were inserted in the maxillary sinus between the elevated Schneiderian membrane and the bony floor of the sinus. A number of follow-up publications and a prospective clinical study demonstrated successful remodeling of the graft material, thereby establishing sinus augmentation with tissue-engineered bone as a possible option for overcoming current limitations of autogenous bone grafting in the posterior maxilla (Schimming and Schmelzeisen 2004, Schmelzeisen et al. 2003).
Implant placement can occur at the same surgical appointment (immediate placement) or following a healing period (delayed placement) depending on the remaining bone height. It is generally acknowledged that for an immediate placement, at least 4 to 5 mm of remaining ridge height is necessary to achieve sufficient immobilization of implants during maturation of the sinus graft (Jensen et al. 1998).
- Insufficient bone height in the posterior maxilla for dental implant placement
- Need for a large amount of autogenous bone grafting material
- Patient’s refusal to have a bone graft from a source that is not his or her own
- Presence of uncontrolled diabetes, immune diseases, or other contraindicating systemic conditions
- Thrombocytopenia or allergically induced thrombocytopenia (type II)
- Radiation therapy to the head and neck area 12 months before proposed surgical treatment
- Chemotherapy in the 12 months before proposed surgical treatment
- Active sinus infection or a history of persistent sinus infections
- Hypersensitivity to bovine albumin, penicillin, gentamicin, amphotericin B
- Excessive smoking habit
- Alcohol and drug abuse
- Physical and psychological handicaps
- Pregnancy and lactating patients
1. For the harvesting procedure, a basic surgical kit such as the one described in Practical Periodontal Plastic Surgery (Dibart and Karima) and an osseous coagulum collector (Citagenix Inc., Laval, Quebec, Canada) can be used.
2. For the sinus augmentation procedure, a basic surgical kit and the following can be used:
- Angulated elevation instruments for separation of the Schneiderian membrane from the inner bony surface of the maxillary sinus (Hu-Friedy, Chicago, IL, USA)
- CollaTape (Zimmer Dental, Carlsbad, CA, USA)
- Bio-Oss (Osteohealth, Shirley, NY, USA)
- Resorbable membrane (Bio-Guide; Osteohealth; and RCM, Bicon, Boston, MA, USA)
SINUS AUGMENTATION USING TISSUE-ENGINEERED BONE DISCS
Technique
Sinus augmentation using tissue-engineered bone requires two surgical procedures: harvesting and transplant implantation surgery.
Harvesting Procedure
Periosteal tissue can be obtained from several locations in the oral cavity. However, access to the lateral cortex of the mandibular body in the apical region of the first molar area is relatively easy and not too invasive. After administering local anesthesia, an intrasulcular or intravestibular incision parallel to the mucogingival junction is made using a No. 15 blade. The incision on the buccal side of the first mandibular molar should extend at least one and one-half teeth toward the anterior and posterior in order to obtain sufficient access. A partial thickness flap is elevated to expose the underlying periosteum. After outlining the area with a No. 15 blade, the periosteal biopsy (approximately 1 cm2) can be collected using a back action chisel or osseous coagulum collector (Fig. 11.1).
Fig. 11.1. Collection of a periosteal biopsy. Periosteal tissue was harvested from the goniac angle of the mandible.
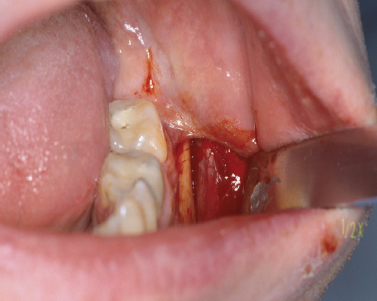
Alternatively, an alveolar bone biopsy (8 × 10 × 2 mm) can be taken from the same side or the tuberosity area after exposing the bone using a distal wedge incision. The collected tissue biopsy needs to be stored in appropriate sterile tissue containers and transferred to an in vitro cell/tissue facility for further culturing and tissue expansion. In addition, a blood sample (approximately 126 mL of blood will be sufficient for 10 tissue-engineered discs) needs to be taken from the patient. This blood sample will be used to produce serum, which is essential for future culturing of the isolated periosteal cells (Schimming and Schmelzeisen 2004). The donor side can be sutured with either resorbable (5-0 chromic gut) or nonresorbable (5-0 silk) suture material.
Postoperative Management
Pain medications should be prescribed as needed. In addition, chlorhexidine rinses twice a day for 21 days starting 1 day after the surgery should be included in the postoperative regimen.
Treatment and Expansion of Periosteal Biopsies
The periosteal tissue biopsy can be cultured using a tissue engineering protocol described by Schmelzeisen and colleagues (2003). In addition, commercial companies such as Bio Tissue Technology, Freiburg, Germany, offer to overtake laborious cell culturing and provide the clinician with the finished tissue-engineered bone discs.
The periosteum needs to be enzymatically digested to isolate progenitor cells. Collagenase CLSII (Clostridium histolyticum) at a concentration of 333 U/mL (Biochrom, Cambridge, UK) in 1:1 DMEM/Ham’s F-12 (Dulbecco’s modified Eagle’s medium; Invitrogen, Carlsbad, CA, USA) can be used, and the resulting cell suspension needs to be washed with phosphate-buffered saline (PBS; Invitrogen). Cells are counted using a hemocytometer and stained with Trypan blue dye to determine the overall cell viability. Afterward, they are resus-pended in 1:1 DMEM/Ham’s F-12 supplemented with 10% autologous serum and seeded into cell culture flasks. The flasks are cultured in a cell culture incubator adjusted to 37°C, 3.5% CO2, and 95% humidity. The medium needs to be replaced every 2 days until cells reach a 70% confluency. At this point, cells are trypsinized (0.02% trypsin and 0.02% EDTA in PBS) for 5 minutes and seeded at a density of 5000/mm2. This step needs to be repeated four times to increase cell number. Following trypsinization, cells are now ready to be incorporated into the transplant discs (Perka et al. 2000). Several scaffold materials such as synthetic and natural polymers, composites, and ceramics have been tested in recent years (Sittinger et al. 2004). />
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


