11
Periodontal diseases in children
P.A. Heasman and P.J. Waterhouse
Chapter contents
11.2 Anatomy of the periodontium in children
11.3 Acute gingival conditions
11.3.1 Primary herpetic gingivostomatitis
11.3.2 Necrotizing ulcerative gingivitis
11.5 Drug-induced gingival enlargement
11.5.4 Clinical features of gingival enlargement
11.5.5 Management of gingival enlargement
11.6 Traumatic gingivitis (gingivitis artefacta and factitious gingivitis)
11.7 Mucogingival problems in children
11.9 Screening for periodontal diseases in children
11.10 Risk factors for periodontal conditions and diseases
11.11 Periodontal complications of orthodontic treatment
11.11.3 Attachment and bone loss
11.12 Aggressive periodontal diseases
11.12.3 Genetic factors and aggressive diseases
11.13 Periodontitis as a manifestation of systemic disease
11.13.1 Papillon–Lefèvre syndrome (PLS)
11.13.3 Chediak–Higashi syndrome
11.13.4 Leucocyte adhesion deficiency syndrome (LAD)
11.13.5 Ehlers–Danlos syndrome
11.13.6 Langerhans’ cell histiocytosis (LCH)
11.1 Introduction
Periodontal diseases comprise a group of infections that affect the supporting structures of the teeth: marginal and attached gingiva, periodontal ligament, cementum, and alveolar bone.
Acute gingival diseases—primarily herpetic gingivostomatitis and necrotizing gingivitis—are ulcerative conditions which result from specific viral and bacterial infection. Chronic gingivitis, however, is a nonspecific inflammatory lesion of the marginal gingiva which reflects the bacterial challenge to the host when dental plaque accumulates in the gingival crevice. The development of chronic gingivitis is enhanced when routine oral hygiene practices are impaired. Chronic gingivitis is reversible if effective plaque control measures are introduced. If left untreated, the condition invariably converts to chronic periodontitis which is characterized by resorption of the supporting connective tissue attachment and apical migration of the junctional epithelia. Slowly progressing chronic periodontitis affects most of the adult population to a greater or lesser extent, although the early stages of the disease are detected in adolescents.
Children are also susceptible to aggressive periodontal diseases that involve both the primary and permanent dentitions and present in localized or generalized forms. These conditions, which are distinct clinical entities affecting otherwise healthy children, must be differentiated from the extensive periodontal destruction that is associated with certain systemic diseases, degenerative disorders, and congenital syndromes.
Periodontal tissues are also susceptible to changes that are not, primarily, of an infectious nature. Factitious stomatitis is characterized by self-inflicted trauma to oral soft tissues, and the gingiva are invariably involved. Drug-induced gingival enlargement is becoming increasingly more prevalent with the widespread use of organ transplant procedures and the use of long-term immunosuppressant therapy. Localized enlargement may occur as a gingival complication of orthodontic treatment.
Table 11.1 A classification of periodontal diseases in children
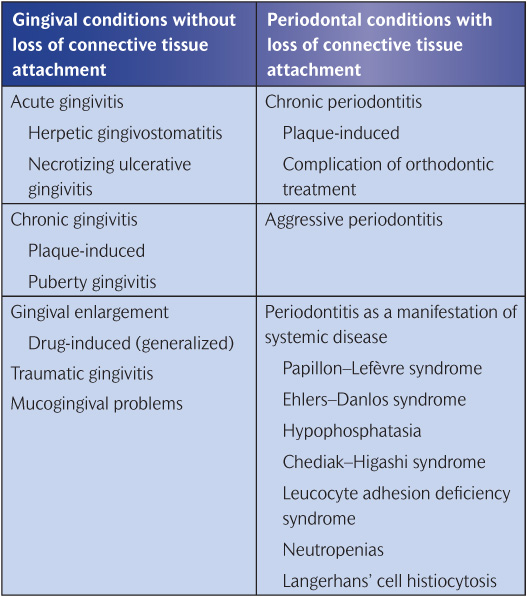
A classification of periodontal diseases in children is given in Table 11.1.
11.2 Anatomy of the periodontium in children
Marginal gingival tissues around the primary dentition are more highly vascular and contain fewer connective tissue fibres than tissues around the permanent teeth. The epithelia are thinner with a lesser degree of keratinization, giving an appearance of increased redness that may be interpreted as mild inflammation. Furthermore, the localized hyperaemia that accompanies eruption of the primary dentition can persist, leading to swollen and rounded interproximal papillae and a depth of gingival sulcus exceeding 3mm.
During eruption of the permanent teeth the junctional epithelium migrates apically from the incisal or occlusal surface towards the cemento-enamel junction (CEJ). While the epithelial attachment is above the line of maximum crown convexity, the gingival sulcus depth often exceeds 6 or 7mm, which favours the accumulation of plaque. When the teeth are fully erupted, there continues to be an apical shift of junctional epithelium and the free gingival margins. Stability of the gingiva is achieved at about 12 years for mandibular incisors, canines, second premolars, and first molars. The tissues around the remaining teeth continue to recede slowly until about 16 years. Thus the gingival margins are frequently at different levels on adjacent teeth that are at different stages of eruption. This sometimes gives the erroneous appearance that gingival recession has occurred around those teeth that have been in the mouth longest.
A variation in sulcus depths around posterior teeth in the mixed dentition is common. For example, sulcus depths on the mesial aspects of Es and 6s are greater than those on the distal of Ds and Es, respectively. This is accountable to the discrepancy in the horizontal position of adjacent CEJs because of the difference in the occluso-apical widths of adjacent molar crowns.
The attached gingiva extends from the free gingival margin to the mucogingival line minus the sulcus depth in the absence of inflammation. Attached gingiva is necessary to maintain sulcus depth, to resist functional stresses during mastication, and to resist tensional stress by acting as a buffer between the mobile gingival margin and the loosely structured alveolar mucosa. The width of attached gingiva is less
The periodontal ligament space is wider in children, partly as a consequence of thinner cementum and alveolar cortical plates. The ligament is less fibrous and more vascular. Alveolar bone has larger marrow spaces, greater vascularity, and fewer trabeculae than adult tissues, features that may enhance the rate of progression of periodontal disease when it affects the primary dentition.
The radiographic distance between the CEJ and the healthy alveolar bone crest for primary canine and molar teeth ranges from 0 to 2mm. Individual surfaces display distances of up to 4mm when adjacent permanent or primary teeth are erupting or exfoliating, respectively, and eruptive and maturation changes must be considered when radiographs are used to diagnose periodontal disease in children. When such changes are excluded, a CEJ-alveolar crest distance of more than 2mm should arouse suspicion of pathological bone loss in the primary dentition. (See Key Points 11.1.)
11.3 Acute gingival conditions
The principal acute gingival conditions that affect children are primary herpetic gingivostomatitis and necrotizing ulcerative gingivitis. The latter is most frequently seen in young adults, but it also affects teenagers.
11.3.1 Primary herpetic gingivostomatitis
Herpetic gingivostomatitis is an acute infectious disease caused by Her-pesvirus hominis. The primary infection is most frequently seen in children between 2 and 5 years of age, although older age groups can be affected. A degree of immunity is transferred to the newborn from circulating maternal antibodies, so an infection in the first 12 months of life is rare. Almost 100% of urban adult populations are carriers of, and have neutralizing antibodies to, the virus. This acquired immunity suggests that the majority of childhood infections are subclinical.
Transmission of the virus is by droplet infection and the incubation period is about a week. The child develops a febrile illness with a raised temperature of 37.8–38.9° C (100–102° F). Headaches, malaise, oral pain, mild dysphagia, and cervical lymphadenopathy are the common symptoms that accompany the fever and precede the onset of a severe oede-matous marginal gingivitis. Characteristic fluid-filled vesicles appear on the gingiva and other areas such as the tongue, lips, and buccal and palatal mucosa. The vesicles, which have a grey membranous covering, rupture spontaneously after a few hours to leave extremely painful yellowish ulcers with red inflamed margins (Fig. 11.1). The clinical episode runs a course of about 14 days and the oral lesions heal without scarring. Rare but severe complications of the infection are aseptic meningitis and encephalitis.
The clinical features, history, and age group of the affected children are so characteristic that diagnosis is rarely problematic. However, if in doubt smears from recently ruptured vesicles reveal degenerating epithelial cells with intranuclear inclusions. The virus protein also tends to displace the nuclear chromatin to produce enlarged and irregular nuclei.
Herpetic gingivostomatitis does not respond well to active treatment. Bed rest and a soft diet are recommended during the febrile stage and the child should be kept well hydrated. Pyrexia is reduced using a paracetamol suspension and secondary infection of ulcers may be prevented using chlorhexidine. A chlorhexidine mouth rinse (0.2%, two to three times daily) can be used in older children who are able to expectorate, but in younger children (under 6 years of age) a chlorhexidine spray can be used (twice daily) or the solution applied using a sponge swab. In severe cases of herpes simplex infection, systemic aciclovir can be prescribed as a suspension (200mg) swallowed five times daily for 5 days. In children under 2 years the dose is halved. Aciclovir is active against the herpesvirus but is unable to eradicate it completely. The drug is most effective when given at the onset of the infection. (See Key Points 11.2 and 11.3.)
Figure 11.1 Ulcerative stage of primary herpetic gingivostomatitis: (a) palatal gingiva; (b) lower lip mucosa.
After the primary infection the herpesvirus remains dormant in the host’s epithelial cells. Reactivation of the latent virus or re-infection in subjects with acquired immunity occurs in children and adults. Recurrent disease presents as an attenuated intra-oral form of the primary infection or as herpes labialis, i.e. the common ‘cold sore’ on the mucocutaneous border of the lips (Fig. 11.2). Cold sores are treated by applying aciclovir cream (5%) five times daily for about 5 days. To prevent auto-inoculation and spread of the lesions onto hands and the face, children should be discouraged from touching the vesicles.
11.3.2 Necrotizing ulcerative gingivitis
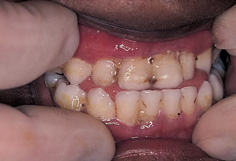
Necrotizing ulcerative gingivitis (NUG) is one of the most common acute diseases of the gingiva. In the USA and Europe, NUG affects young adults in the 16–30 age range with a reported incidence of 0.7–7%. In developing countries, NUG is prevalent in children as young as 1 or 2 years of age, when the infection can be very aggressive leading to extensive destruction of soft and hard tissues (Fig. 11.3). Epidemic-like occurrences of NUG have been reported in groups such as army recruits and first-year college students. These outbreaks are more likely to be a consequence of the prevalence of common predisposing factors rather than communicability of infection between subjects.
Figure 11.2 Herpetic ‘cold sore’ at the vermilion border of the lower lip.
Figure 11.3 A 5-year-old Ethiopian boy with necrotizing ulcerative gingivitis.
Clinical features
NUG is characterized by necrosis and ulceration, which first affect the interdental papillae and then spread to the labial and lingual marginal gingiva. The ulcers are ‘punched out’, covered by a yellowish-grey pseu-domembranous slough, and extremely painful to the touch (Fig. 11.3). The acute exacerbation is often superimposed upon a pre-existing gingivitis, and the tissues bleed profusely on gentle probing. The standard of oral hygiene is usually very poor. A distinctive halitosis is common in established cases of NUG, although fever and lymphadenopathy are less common than in herpetic gingivostomatitis.
The clinical course of NUG is such that the acute stage enters a chronic phase of remission after 5–7 days. However, recurrence of the acute condition is inevitable, and if this acute–chronic cycle is allowed to continue the marginal tissues lose their contour and appear rounded. Eventually, the inflammation and necrosis involve the alveolar crest and the subsequent necrotizing periodontitis leads to rapid bone resorption and gingival recession. Progressive changes are also a consequence of inadequate or incomplete treatment.
Aetiology
A smear taken from an area of necrosis or the surface of an ulcer will reveal numerous dead cells, polymorphonuclear leucocytes, and a sample of the micro-organisms that are frequently associated with NUG. Fusiform bacteria and spirochaetes are both numerous and easy to detect. A fusospirochaetal complex has been strongly implicated as the causative organisms in NUG. Other Gram-negative anaerobic organisms, including Porphyromonas gingivalis, Veillonella species, and Selenomonas species, have been detected, which suggests that NUG could be a broad anaerobic infection.
A viral aetiology has also been suggested, primarily because of the similarity between NUG and known viral diseases. For example, the restriction of the disease to children and young adults may imply that older subjects have undergone seroconversion (and thus are immune) as a consequence of clinical or subclinical viral infection in earlier life. The recurring episodes of the disease may also be explained by a viral hypothesis. The ability to undergo latent infection that is subject to reactivation is a characteristic of the herpesvirus. Therefore the argument for the implication of a virus in NUG is valid and novel, although a specific virus has yet to be isolated from oral lesions.
Predisposing factors
Poor oral hygiene and pre-existing gingivitis invariably reflect the patient’s attitude to oral care. Many young adults with NUG are heavy smokers. The effect of smoking on the gingiva may be mediated through a local irritation or by the vasoconstrictive action of nicotine, thus reducing tissue resistance and making the host more susceptible to anaerobic infection. Smoking is obviously not a predisposing factor in young children. However, children in underdeveloped countries are often undernourished and debilitated, which may predispose to infection. Outbreaks of NUG in groups of subjects who are under stress has implicated emotional status as an important predisposing factor. Elevated plasma levels of corticosteroids as a response to an emotional upset are thought to be a possible mechanism.
It is conceivable that all the predisposing factors have a common action to initiate or potentiate a specific change in the host such as lowering the cell-mediated response. Indeed, patients with NUG have depressed phagocytic activity and chemotactic response of their polymorphonuclear leucocytes. (See Key Points 11.4.)
Treatment
It is important that the patient is informed at the outset of the nature of NUG and the likelihood of recurrence of the condition if the treatment is not completed. Smokers should be advised to reduce the number of cigarettes smoked. A soft multi-tufted brush is recommended when a medium-textured brush is too painful.
Mouth rinses may be recommended but only for short-term use (7-10 days). Rinsing with chlorhexidine (0.2%) for about a minute reduces plaque formation, and the use of a hydrogen peroxide or sodium hydroxyperborate mouth rinse oxygenates and cleanses the necrotic tissues.
Mechanical debridement should be undertaken at the initial visit. An ultrasonic scaler with its accompanying water spray can be effective with minimal discomfort for the patient. Further, if NUG is localized to one part of the mouth, local anaesthesia of the soft tissues can allow some sub-gingival scaling to be undertaken.
In severe cases of NUG, a 3-day course of metronidazole (for children over 10 years of age: 200mg three times daily) alleviates the symptoms, but the patients must be informed that they are required to reattend for further treatment.
Occasionally, it is necessary to surgically recontour the gingival margin (gingivoplasty) to improve tissue architecture and facilitate sub-gingival cleaning. (See Key Points 11.5.)
11.4 Chronic gingivitis
National Surveys (1973, 1983, 1993, and 2003) of children’s dental health in the UK show that the prevalence of chronic gingivitis increases steadily between the ages of 5 and 12 years and is closely associated with the amount of plaque, debris, and calculus present (Fig. 11.4). For example, in 2003, 32% of 5-year-olds had some signs of gingivitis, and the proportion increased to 65% at the age of 12. The prevalence of gingivitis peaks at about 12 years (65%) and then decreases slightly with age to 15 years (52%). In terms of gingivitis, there has been no improvement over the decades between surveys. Indeed, in 2003, 13–19% more children of all ages between 5 and 12 years had signs of gingivitis when compared with 1983. However, these differences were not maintained with increasing age, as 52% of 15-year-olds had gingivitis in both 1993 and 2003. These data suggest that the gingival condition of children in the UK has deteriorated over the 20 years between 1983 and 2003. Certainly, changes in gingival health do not mirror the dramatic improvement in the prevalence of caries over the same period. Children’s mouths tended to be cleaner in 1983 than in 1973. This trend was reversed by 1993 when 10–20% more children of all ages had plaque deposits, and in 2003 this had deteriorated even further (by 5–6%) across all age groups. Levels of calculus in 8-, 12-, and 15-year-olds increased between 1993 and 2003.
Figure 11.4 Chronic marginal gingivitis in a 10-year-old girl.
The onset of puberty and the increase in circulating levels of sex hormones is one explanation for the increase in gingivitis seen in 11-year-olds. Oestrogen increases the cellularity of tissues and progesterone increases the permeability of the gingival vasculature. Oestradiol also provides suitable growth conditions for species of black pigmenting organisms which are associated with established gingivitis.
Histopathology
The inflammatory infiltrate associated with marginal gingivitis in children is analogous to that seen in adults during the early stages of gingival inflammation. The dominant cell is the lymphocyte, although small numbers of plasma cells, macrophages, and neutrophils are in evidence. There is a relative absence of plasma cells, which are found in abundance in more established and advanced lesions in adults.
Microbiology
The first organisms to colonize clean tooth surfaces are the periodontal harmless Gram-positive cocci. A more complex flora of filamentous and fusiform organisms indicates a conversion to a Gram-negative infection which, when established, comprises significant numbers of Capnocytophaga, Selenomonas, Leptotrichia, Porphyromonas and Spi-rochaete species. These species are also cultivable from established and advanced periodontal lesions in cases of adult periodontitis.
The role of gingival inflammation
It is well established that gingivitis is a precursor of chronic periodontitis, although the role that gingival inflammation may have in predicting the development of chronic periodontitis may have been understated. There is little evidence in the literature to implicate specific bacteria in predicting when gingivitis will progress to a state of chronic attachment loss and a cause-and-effect relationship is certainly not clear. Indeed, the inflammatory lesion itself may be crucial in changing the composition of the sub-gingival microflora, a concept that is consistent with evidence from long-term epidemiological studies which shows that the severity of gingivitis rather than the presence of specific pathogens is the more reliable predictor of long-term tooth loss. (See Key Points 11.6.)
Home-based oral healthcare
The treatment and prevention of chronic plaque-induced gingivitis are dependent on achieving and maintaining a standard of plaque control which, on an individual basis, is compatible with health. Toothbrushing is the principal method of removing dental plaque.
Toothbrushing should start as soon as the first primary tooth erupts, and it is recommended that teeth are brushed twice daily with a smear or small pea-sized amount of fluoride toothpaste. Children who are younger than 3 years should have their teeth brushed by an adult using toothpaste containing no less than 1000ppm fluoride. If older than 3 years, toothpaste containing 1350-1500ppm fluoride is recommended.
Data from the Child Dental Health Survey 2003 showed that an adult carer either brushed or helped with brushing the teeth for approximately 50% of 5-year-olds and 15% of 8-year-olds. Within the group of 5-year-olds who brushed their own teeth without adult help, a significantly greater proportion had plaque compared with those children receiving adult help. It is generally recommended that toothbrushing should be supervised until the child is at least 7 years old or until their manual dexterity is sufficiently developed.
The Child Dental Health Survey 2003 also revealed that the use of powered toothbrushes was widespread (50-75% of all children reported using them). Powered toothbrushes now provide a widely available alternative to the more conventional manual toothbrushes for cleaning teeth. There is considerable evidence in the literature to suggest that powered toothbrushes are beneficial for specific groups: patients with fixed orthodontic appliances, for whom there is also evidence that powered toothbrushes are effective in reducing decalcification; children and adolescents; and children with special needs. It remains questionable whether children who are already highly motivated with respect to tooth-cleaning will benefit from using a powered toothbrush. It is possible that, particularly in children, any improved plaque control as a consequence of using a powered toothbrush may result from the ‘novelty effect’ of using a new toothbrush rather than because the powered toothbrush is a more effective cleaning device.
A systematic review evaluating manual and powered toothbrushes with respect to oral health has made some important conclusions. Compared with manual toothbrushes, rotating/oscillating designs of powered toothbrushes reduced plaque and gingivitis by 7-17%, although the clinical significance of this could not be determined. Therefore powered brushes are at least as effective and equally as safe as their manual counterparts with no evidence of increased incidence of soft tissue abrasions or trauma. No clinical trials have looked at the durability, reliability, and relative cost-effectiveness of powered and manual brushes, so it is not possible to make any recommendation regarding overall toothbrush superiority.
The use of dental floss by children is not widespread, although floss-holders specifically designed for children’s use are available. It is recommended that flossing in young children is supe/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


 Key Points 11.1
Key Points 11.1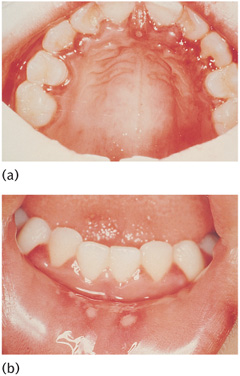
 Key Points 11.2
Key Points 11.2 Key Points 11.3
Key Points 11.3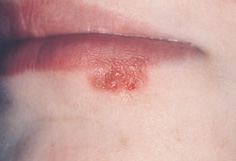
 Key Points 11.4
Key Points 11.4 Key Points 11.5
Key Points 11.5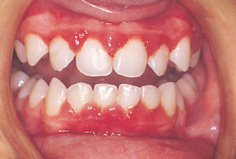
 Key Points 11.6
Key Points 11.6