CHAPTER 11
Biologic Growth Factors and Bone Morphogens in Bone Regeneration Procedures
Perhaps the most promising research currently underway on bone regeneration is the incorporation of biologic growth factors and bone morphogens. In general, these biologic proteins are intimately involved in regulating the cellular events that occur during repair of bone and other tissues in the body. Such processes include cell proliferation, chemotaxis, mitosis, differentiation, and matrix synthesis. Growth factors bind to specific receptors on target cell surfaces, leading to a complex cascade of intracellular events that culminates in bone formation. The basic theory is that by clinically applying an added number of these bone-building “orchestrators” to a wound site, they may in effect jump-start and perhaps even improve the body’s normal osteoregenerative potential. Some researchers even speculate that in the not-too-distant future, growth factors and bone morphogens may eventually make the use of other bone graft materials—even autogenous bone—obsolete. The potential also exists for achieving earlier and better osseointegration of dental implants.
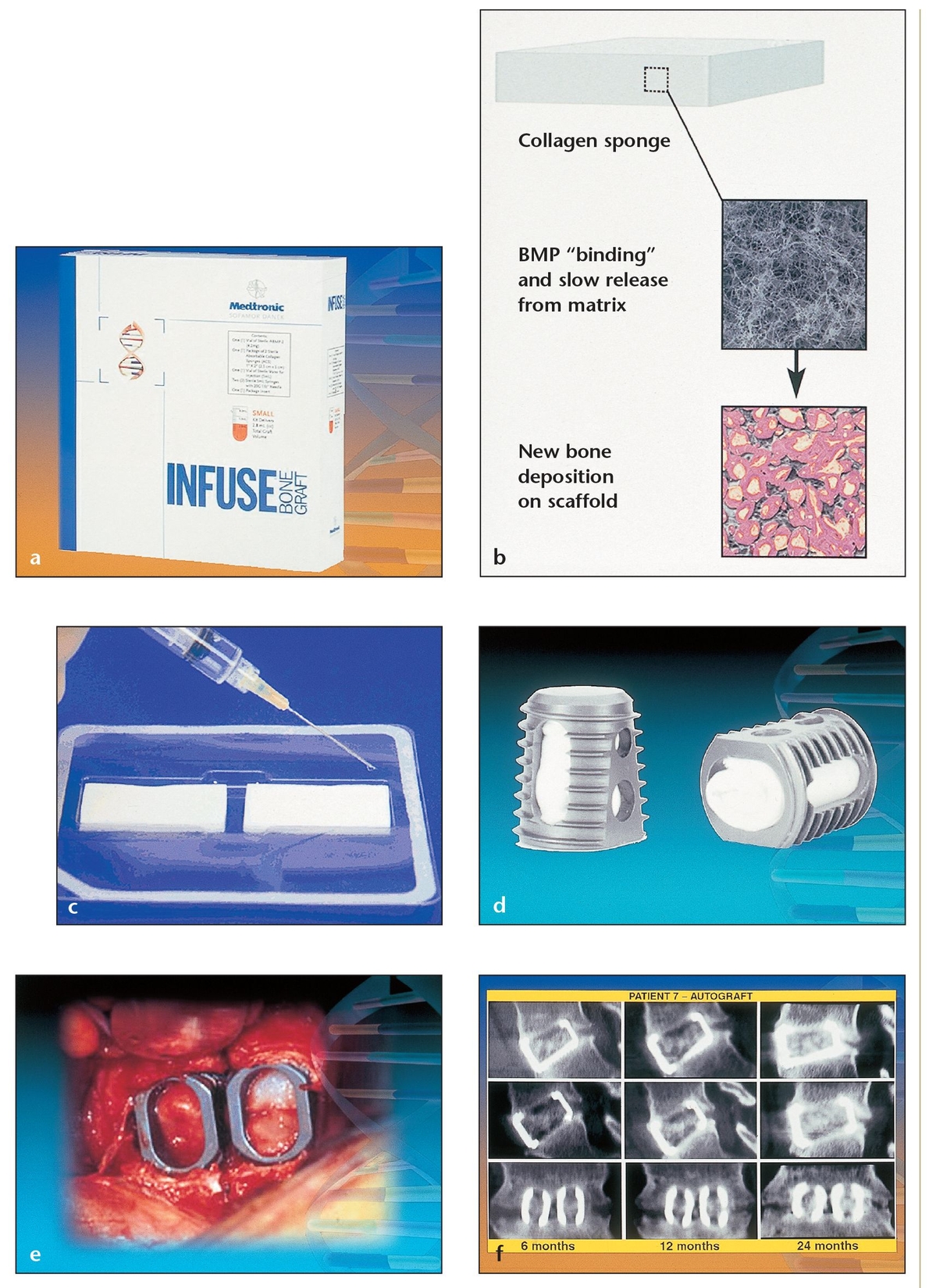
(a) Small package of the InFuse material, which contains two collagen sponges and rhBMP-2.
(b) When used as a carrier for rhBMP-2, an absorbable collagen sponge retains the rhBMP-2 at the surgical site and provides a favorable environment for bone formation.
(c) Absorbable collagen sponges act as carriers to deliver rhBMP-2.
(d) Tapered interbody titanium cages filled with autogenous iliac crest bone grafts or with collagen sponges soaked in rhBMP-2 are used for lumbar spine surgery in patients with degenerative lumbar disc disease.
(e) Two titanium cages filled with the rhBMP-2–saturated collagen sponges have been placed at the spinal surgical site. This comparative clinical study randomly divided the subjects into two groups that underwent interbody fusion via two tapered fusion cages: one group of 143 patients with rhBMP-2 on an absorbable collagen sponge and the other of 132 patients with autogenous anterior iliac crest grafts. At 24 months, the fusion rate was 5.8% higher in the BMP group (94.5% fusion rate) than in the control group (88.7% fusion rate).
(f) A serial computerized tomography (CT) image shows the right cage (upper), the left cage (middle), and a coronal view of both (lower) at 6, 12, and 24 months, revealing evidence of bony induction and early incorporation of bone in both groups.
Such biologic treatments are just beginning to enter the market for orthopedic applications. InFuse Bone Graft (Medtronic Sofamore Danek, Memphis, TN) received Food and Drug Administration (FDA) approval in early 2002 for use in spinal fusion surgery. This product consists of two collagen sponges that can be soaked in recombinant human bone morphogenetic protein-2 (rhBMP-2) and placed into the defect or inserted into a titanium cage for spinal surgery (Fig 11-1). Another biologic product, Stryker Biotech OP-1 (Stryker Biotech, Natick, MA), received a Humanitarian Device Exemption from the FDA in 2001 for use in no more than 4,000 adult cases per year requiring treatment for nonunions of tibia secondary to trauma in which autografts have failed or are not feasible. The product was also approved in 2001 by the European Agency for Evaluation of Medicinal Products for the same application, without limitation on number of cases, in all 15 European Union countries plus Iceland and Norway. Thus far no biologic treatments have been approved for maxillofacial reconstruction; however, as of this writing a large, multi-center, phase 3 clinical trial is underway on rhBMP-2, one of the most promising growth factors for use in dental applications.
Several other individual growth factors and their specific functions in bone healing have also been identified and tested for their ability to affect bone growth. These include platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), fibroblastic growth factor (FGF), and transforming growth factor-beta (TGF-β). This chapter describes their function in bone formation and provides information on the current state of research for each factor.
Growth Factors
Platelet-Derived Growth Factor
PDGF is one of the principal wound-healing hormones. It plays several important roles in bone formation and regeneration, including (1) increasing the number of healing cells (including osteoblasts) present at the wound site; (2) transforming endothelial mitoses into functioning capillaries; (3) debriding the wound site; and (4) providing a second-phase source of growth factors for continued bone regeneration.1 Its primary effect is as a potent mitogen, initiating cell division, and as a chemotactic factor for cells of mesenchymal origin, including osteoblasts. Several subtypes of PDGFs exist; they consist of homodimers or heterodimers of the PDGF-A and PDGF-B gene products. Sources of PDGF include platelets as well as activated macrophages and bone matrix.2 The most intense area of PDGF research involves the use of platelet-rich-plasma (PRP), as described below.
Because growth factors are cell-specific, meaning that particular growth factors work only to stimulate specific cell types, many researchers studying the usefulness of PDGF have also focused on combining it with other growth factors to optimize complete tissue regeneration. Much of the research on such PDGF combinations—particularly with IGF—has addressed their usefulness for periodontal regeneration,3 including a recent clinical trial (FDA phase 1 and 2) of 38 patients with moderate to severe periodontitis. At 6 to 9 months postsurgery, patients treated with a combination of PDGF-BB and IGF-1 (150 µg/mL of each) experienced a statistically significant 43% mean bone fill (with 2.1 mm vertical bone height), whereas control groups receiving either sham surgery or carrier alone experienced an average 18.5% fill (with 0.8 mm vertical bone height).4
Lee et al demonstrated the ability of PDGF to induce bone regeneration earlier than guided bone regeneration alone.5 His team placed 500 ng of PDGF-BB in molded poly(L-lactide) membranes and inserted them into defects of various sizes in four rabbit calvaria. At 4 weeks, the dome-shaped treated membranes obtained almost complete bone formation (28% new bone) compared with untreated membranes (13% new bone). Controls did not completely refill until 12 to 18 weeks.
In a few canine studies, researchers have demonstrated that the PDGF-IGF combination may also enhance bone growth around implants and accelerate osseointegration. In a study by Lynch et al, 40 implants with apical holes containing either the PDGF-IGF combination or carrier alone were assessed after 1 and 3 weeks in the mandibular premolars of eight beagle dogs.6 At 1 week, peri-implant bone fill and bone-to-implant contact were significantly higher in the test group. By 3 weeks, bone fill was still significantly greater in the test group, but bone-to-implant contact differences became insignificant. In another study, Stefani et al also noted significantly earlier bone formation and more bone-to-implant contact (22% vs 17%) within the first 3 weeks in patients treated with the PDGF-IGF combination compared with controls in a histometric study involving immediate implant placement in eight dogs.7 Yet another study evaluated the PDGF-IGF combination with guided bone regeneration in fresh extraction sockets of four dogs with buccal dehiscences and implants. 8 At 18 weeks, Becker et al observed denser bone and twice as much bone-to-implant contact and area of peri-implant bone fill in the defects treated with the growth factors and expanded polytetrafluoroethylene (e-PTFE) membrane compared with controls receiving membranes alone.8
The mechanism by which these growth factor combinations improve bone and periodontal regeneration still must be proven in vivo, as must the specific amounts of the growth factors that would be optimally useful.
Insulin-like Growth Factor
There are two types of IGF—IGF-I and IGF-II—that function similarly but are independently regulated. As their name indicates, IGFs are biochemically and functionally similar to insulin.1 They are primarily produced by the liver and circulate in the vascular system.2 Although its direct or indirect effects on bone remodeling are not fully understood, IGF-I appears to stimulate bone formation by increasing cellular proliferation and differentiation as well as bone matrix production. Some evidence suggests IGF-I may mediate the ability of parathyroid hormone to stimulate osteoprogenitor cell proliferation within bones.9
As mentioned previously, animal studies suggest that IGF-I works synergistically with PDGF; it initiates better tissue regenerative results in combination than it does on its own. Thus, most in vivo studies have incorporated IGF in combination with this and other growth factors.
Fibroblast Growth Factor
FGFs received their name because of their general growth-promoting effects on most fibroblastic cell types. This growth factor—which occurs in both acidic and basic forms and is stored in bone—also stimulates angiogenesis for vascular invasion of bone, wound healing, and cell migration. Both forms of FGF stimulate bone cell replication, but they can also inhibit matrix synthesis by bone cells under some conditions, and they have no stimulatory effect on mature osteoblasts.3, 10
Lab studies suggest FGFs may stimulate endothelial and periodontal ligament cell migration and proliferation as well, but only a few positive in vivo studies supporting an enhanced bone regenerative benefit for either orthopedic or craniomaxillofa-cial applications have been reported.11, 12, 13, 14 Appropriate dosage and delivery systems are important unresolved issues regarding FGF.13, 14, 15
Transforming Growth Factor-Beta
TGF-β is a multifunctional growth factor synthesized by many cell types, and almost every cell type can be stimulated by at least one of the various TGF-β molecules. It is one of the major growth factors present in bone (as well as platelets) and is structurally related to, but functionally different from, the BMPs. Generally, TGF-β is a weak mitogen for osteoblasts. It has been shown to be chemotactic for bone cells and may increase or decrease their proliferation depending on various conditions. It has also been shown to stimulate type-1 collagen synthesis.2,3 Several in vivo studies have shown that TGF-β can induce new cartilage and bone, but only if it is planted in proximity to a bony site.3 As with IGF, in vitro evidence suggests that TGF-β may exert more powerful bone-forming effects in combination with PDGF.16 In vivo studies demonstrate that TGF-β1 can induce bone closure of calvar-ial defects in rabbits,17 enhance fracture healing in rabbit tibiae,18 and promote osseous wound healing in rats.19 These studies also suggest that TGF-β1 has a dose-related effect on bone induction, but that a higher dose does not always generate more bone.
Two recent animal studies suggest that TGF-β1 maybe a useful adjunct to guided tissue regeneration (GTR) in inducing greater early bone formation. Mohammed et al studied TGF-β1 with GTR in Class II furcation defects in the mandibular premolars of 24 sheep.20 Defects treated with TGF-β1 and membrane had significantly greater mean bone volume at 6 weeks (59%) than did those treated with membrane (53%) or carrier alone (43%). Ruskin et al also noted statistically more bone formation at 8 weeks in surgically created alveolar defects in 13 foxhounds when TGF-β1 and a membrane were used (84%) compared with when TGF-β1 (61%) or a carrier alone (30%) were used.21
Platelet-Rich Plasma
Although the application of growth factors shows great promise for enhancing wound healing and bone regeneration, approved pharmaceutical formulations are still not forthcoming. In addition, procuring growth factors for eventual clinical use is likely to be an expensive proposition. Therefore, as an alternative source of growth factors, PRP has become an increasingly popular clinical tool for several types of surgery, including oral bone-regenerative procedures.
PRP is a concentrated autologous source of several growth factors, particularly PDGF, TGF-β1, and TGF-β2, as well as vascular endothelial growth factor (VEGF), IGF, and other growth factors possibly contained within platelets. Once the clinician prepares PRP by extracting a small amount of the patient’s own blood and then sequestering and concentrating the platelets—a process that requires 20 to 30 minutes in an outpatient clinical setting—PRP can be used to enhance graft material, to form a growth factor–rich membrane, 22 or to enhance a traditional barrier membrane. PRP’s fibrinogen component also makes it an excellent hemostatic tool, tissue sealant, wound stabilizer, and graft condenser through the creation of a gel-like substance that allows for sculpting and excellent adherence in defects.
It has been shown radiographically that adding PRP to graft material significantly accelerates the rate of bone formation and improves trabecular bone density as compared with sites treated only with autogenous graft material.22, 23 Widespread clinical reports and recent published research also suggest that PRP significantly enhances soft tissue healing; reduces bleeding, edema, and scarring; and decreases patients’ self-reported pain levels postoperatively. 24, 25, 26, 27, 28, 29 Some evidence even suggests that adding PRP to graft material leads to growth of bone that is more dense than native bone,23, 30 a potential benefit that has not been reported in studies where BMPs or growth factors are applied alone. PRP growth factors are particularly attractive for use in patients with conditions that typically reduce the success of bone grafts and osseointegration, including those with an edentulous and severely atrophic maxilla, osteoporosis, and tissues that have been scarred and altered by prior dental disease.31
The concentrated levels of PDGF and TGF-β in PRP (in addition to the presence of other growth factors and proteins) appear to provide benefits that lead to more rapid and effective bone regeneration. The beneficial effects of these growth factors can perhaps best be understood through a description of the components and biology of PRP.
PRP components and bone healing
An understanding of how PRP growth factors may enhance bone grafts and implant osseointegration requires a description of the components of PRP and their roles in wound and bone healing (Fig 11-2).
Specific studies of PRP have identified at least three important factors in the alpha granules of the sequestered platelets: PDGF, TGF-β1, and TGF-β2.32, 33, 34 In addition, other studies have documented the presence of VEGF and IGF-I in platelets from peripheral human blood tests.35, 36, 37, 38, 39
PDGF is considered one of the principal healing hormones in any wound, and platelets are the greatest source of this growth factor in the human body.23 PDGF isolated from human platelets consists of homodimers (AA or BB) or heterodimers (AB) of the two PDGF gene products, with the heterodimers predominating. Two different PDGF homodimers, PDGF-AA and -BB, are 56% autologous, encoded by different genes, and independently regulated. Recent evidence indicates that the PDGF-AB heterodimer and PDGF-BB ho-modimer have equivalent activity and are equally potent in stimulating DNA synthesis in human fibroblasts.40, 41
PDGF-AA and PDGF-BB were found to be potent mitogens for human periodontal ligament cells, maximally enhancing the mitogenic response 10- and 12-fold, respectively; furthermore, the mitogenic response for both PDGF isoforms increased, depending on the dosage. A 1989 study identifies two receptor subunit molecules, alpha and beta, for PDGF.42 The AA isoform binds only alpha-receptor dimers, while the BB isoform binds all combinations of alpha- and beta-receptor dimers. The number of receptor-binding sites for each isoform is thought to determine the mitogenic potency of each isoform in a given cell type. Thus, the strategy behind PRP use is amplification and acceleration of the effects of growth factors contained in platelets, the universal initiators of almost all wound healing (Fig 11-3).
TGF-β1 and -β2 are multifunctional cytokines involved in general connective tissue repair and bone regeneration. Their most important role appears to be the chemotaxis and mitogenesis of osteoblast precursors and the stimulation of deposition of collagen matrix for wound healing and bone formation.23 These growth factors also enhance bone formation by increasing the rate of stem cell proliferation and, to some degree, inhibit osteoclast formation and, thus, bone resorption.43, 44 Bone and platelets contain approximately 100 times more TGF-β than do any other tissues, and osteoblasts contain the greatest number of TGF-β receptors.45
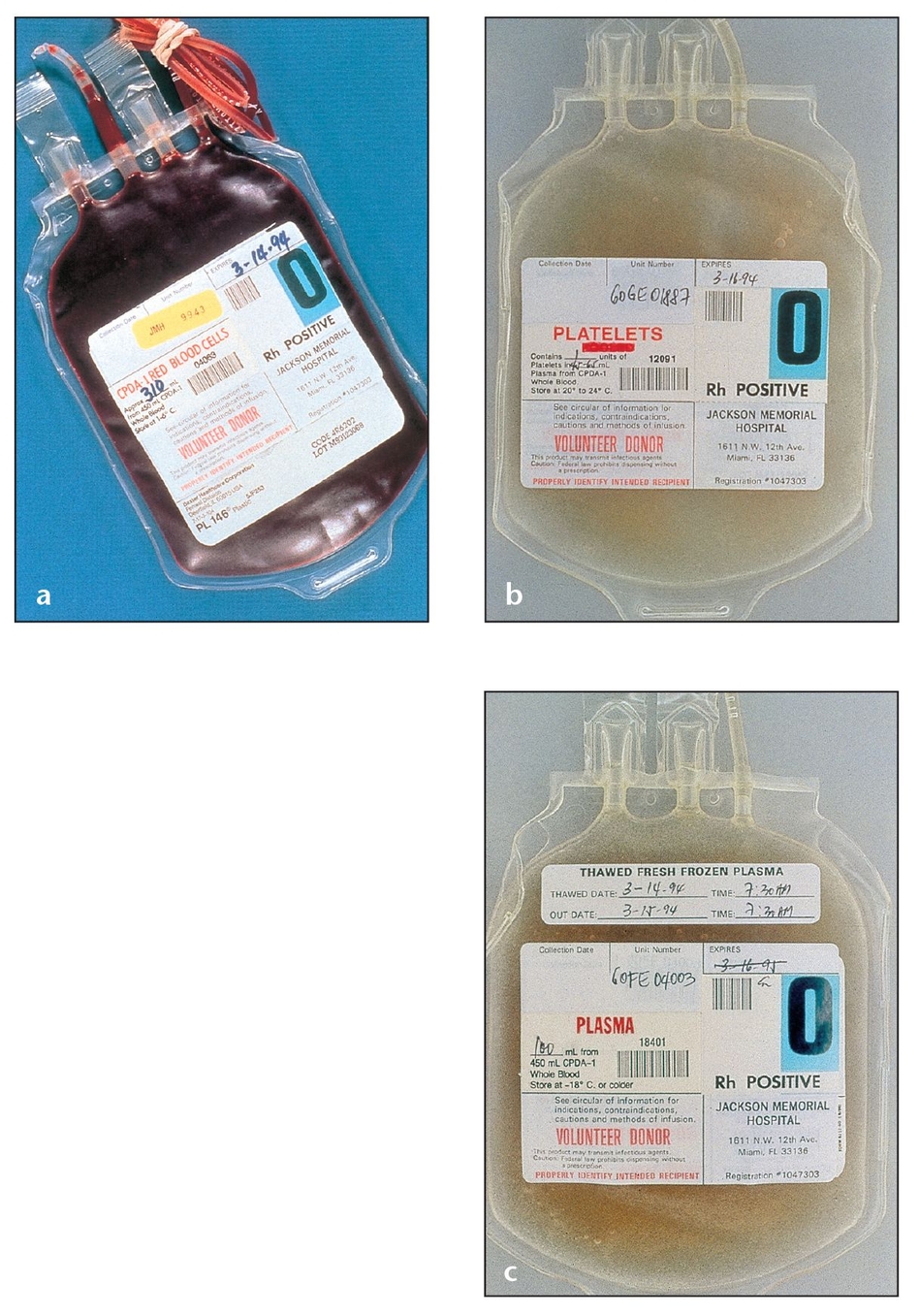
(a) Proper technique preserves cell viability when whole blood is separated into its main components, such as these packed red blood cells from a blood bank.
(b) A bag of concentrated platelets from a blood bank.
(c) A bag of blood plasma from a blood bank.
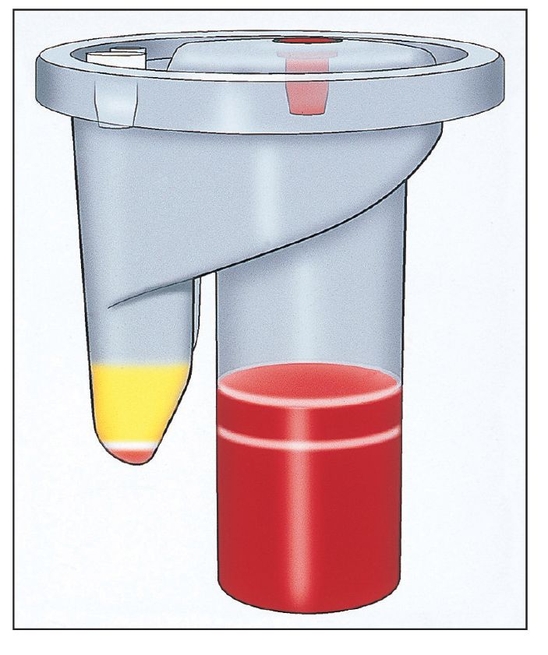
A device designed and priced for office use separates 20 to 60 mL of blood drawn from a patient into its components.
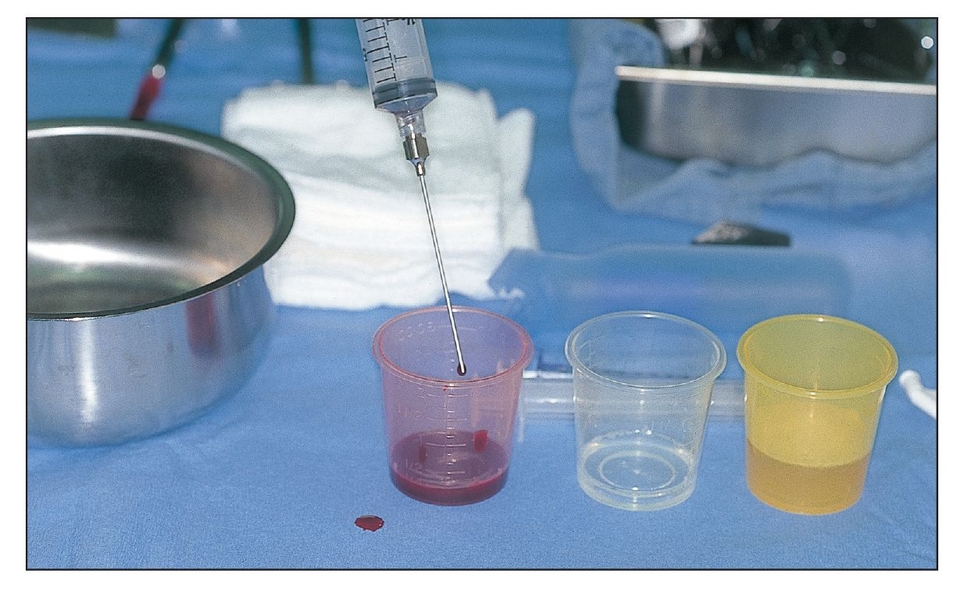
PRP is placed on the surgical field. A mixture of thrombin and calcium chloride is used to activate the concentrated platelets to release their growth factors and to initiate gelling for better clinical handling.
PRP also contains fibrin, fibronectin, and vitronectin, cell-adhesion moleculesthat support cell migration (osteoconduction) (Fig 11-4).46 The dense fibrin net that PRP provides is also hemostatic, and it helps to bind graft material and to stabilize the blood clot in the defect once applied, possibly acting as a barrier membrane to impede the migration of epithelial and connective tissue cells.46 Its hemostatic and wound-sealing effects have proven useful in cardiovascular, orthopedic, plastic, and other surgeries. PRP also modulates and upregulates one growth factor’s function in the presence of the other growth factors. This feature separates PRP growth factors from recombinant growth factors, which focus only on a single regeneration pathway.22
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


